Rybelsus weight loss in non diabetic patients is compared with different diabetes and weight loss drugs.
It has only been approved for the treatment of diabetes mellitus type 2. Rybelsus has not been approved by the FDA for weight loss in diabetic and non-diabetic patients.
Rybelsus is the oral formulation of Semaglutide, a novel GLP-1 analog. The high-dose Semaglutide, Wegovy, has been approved for overweight and obese diabetic and non-diabetic patients.
What is Rybelsus?
Let’s not confuse Rybelsus with other formulations of Semaglutide. Semaglutide is a GLP-1 analog that has recently been marketed by NOVO-Nordisk after getting approved by the FDA.
It has three formulations, two of which have been approved by the FDA for use in diabetic patients while one is approved only for the treatment of obesity and weight loss.
Semaglutide formulations approved for Diabetes:
- Rybelsus: Oral formulation of Semaglutide, available as tablets.
- Ozempic: Injectable formulation of Semaglutide administered once weekly subcutaneously.
Semaglutide formulations approved for weight loss in diabetics and non-diabetics:
- Wegovy: It is a high-dose Semaglutide formulation administered once weekly as a subcutaneous injection.
Rybelsus is available as a tablet. It is available in three strengths: 3 mg, 7 mg, and 14 mg tablets. It is taken once daily 30 minutes before breakfast for the treatment of diabetes.
Ryebelsus lowers blood glucose by activating GLP-1 receptors, acting as an incretin-mimetic. It enhances the release of insulin in a glucose-dependent mechanism and suppresses appetite.
When taken daily, the maximum drug concentration is achieved after 3 days. It has a half-life of about 5 days and is excreted primarily in the feces.
| You may also like to read: |
Rybelsus for Weight Loss in Non-Diabetic Patients:
Rybelsus is not FDA-approved for the treatment of obesity and weight loss. It is also not FDA-approved for use in non-diabetic patients.
However, like metformin, Rybelsus may be one of the favorite drugs used off-label for the treatment of weight loss in diabetic and non-diabetic individuals.
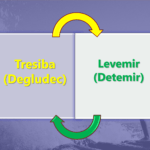
When used in diabetic patients, Rybelsus resulted in clinically significant weight loss compared to placebo in doses of 7 mg and 14 mg.
| Baseline Weight | Weight after 26 weeks | Mean Weight Loss | |
| Placebo | 88.6 | 87.2 | -1.4 kgs |
| Rybelsus 7 mg | 89 | 86.7 | -2.3 kgs |
| Rybelsus 14 mg | 88.1 | 84.4 | -3.7 kgs |
Rybelsus monotherapy compared to placebo resulted in significant weight loss after 26 weeks.
Compared to the placebo, there was a mean difference in weight loss of 2.3 kgs after 26 weeks.
| You may also like to read: |
The weight loss effects of Rybelsus compared with metformin [Ref]:
Metformin is commonly used for weight loss, although it has not been approved for weight loss. It is especially preferred in people with insulin resistance, prediabetes, and women with PCOS.
The weight loss observed with metformin ranges between 2 to 3 kgs. In the DPP (Diabetes Prevention Program), metformin use was associated with a 3.5% reduction in BMI (body mass index).
Rybelsus, on the other hand, has been observed to cause a weight loss ranging from 2.5 to 4 kgs in various clinical trials.
Thus, when used as an off-label medicine for the treatment of obesity, Rybelsus is an alternative, rather a preferred medicine compared to metformin.
| You may also like to read: |
The weight loss effects of Rybelsus compared with Jardiance [Ref]:
Jardiance (empagliflozin) is an SGLT2 inhibitor that is commonly used to treat individuals with diabetes. It is also now used to treat patients with heart failure without diabetes.
Since Jardiance causes the loss of sugars via urine, it is associated with weight loss. It has been observed to cause significant weight loss in some patients. However, it has not been approved by the FDA for weight loss.
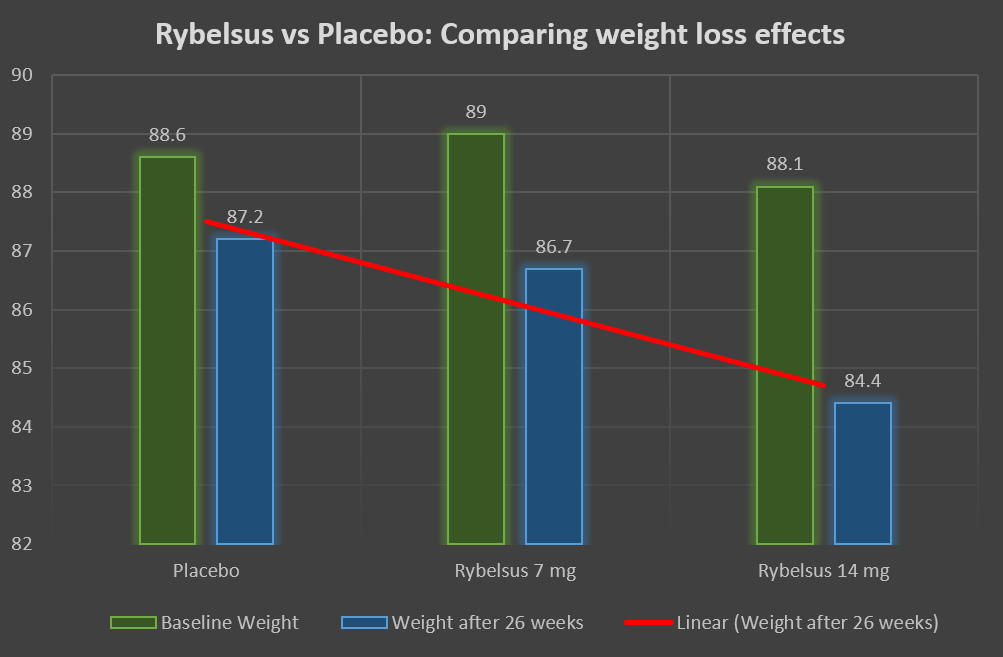
Rybelsus and Jardiance were compared. The two drugs were given as an add-on treatment to metformin.
Rybelsus in a dose of 14 mg resulted in a weight loss of 3.8 kgs from the baseline compared to 3.7 kgs weight loss in the empagliflozin (Jardiance) 25 mg group.
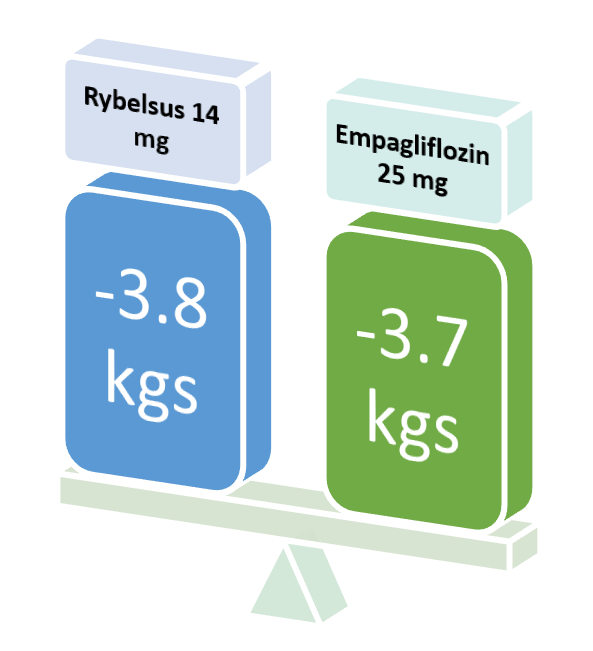
Thus, if not superior, Rybelsus was non-inferior to Jardiance in terms of its weight loss effect.
| You may also like to read: |
Rybelsus vs Januvia for weight loss [Ref]:
Januvia (Sitagliptin) is considered a weight-neutral drug. It belongs to the class of drugs called DPP-IV inhibitors that act by prolonging the action of endogenous incretins.
Rybelsus is a GLP-1 analog and acts as an incretin-mimetic.
The weight loss effects of Rybelsus were compared with Sitagliptin (Januvia). Rybelsus in a dose of 7 mg and 14 mg resulted in significantly greater weight loss compared to Sitagliptin 100 mg.
| Baseline Weight | Weight after 26 weeks | Mean weight loss | |
| Sitagliptin 100 mg | 90.9 | 90.3 | -0.6 kgs |
| Rybelsus 7 mg | 91.3 | 89.1 | -2.2 kgs |
| Rybelsus 14 mg | 91.2 | 88.1 | -3.1 kgs |
The mean weight loss in the Rybelsus groups was significantly greater compared to Sitagliptin (Januvia).

Compared to Januvia, there was a mean of -1.6 kgs and -2.5 kgs greater weight loss in the Rybelsus 7 mg and 14 mg groups respectively.
| You may also like to read: |
Rybelsus vs Liraglutide for weight loss [Ref]:
Until now, we compared Rybelsus with non- FDA-approved medications for weight loss.
However, Liraglutide (Saxenda, Victoza) is an injectable once-daily administered GLP-1 analog that has been approved by the FDA for weight loss.
Before discussing the weight loss effects, here are a few similarities and differences between Rybelsus and Liraglutide:
- Rybelsus (Semaglutide) and Liraglutide are both GLP-1 analogs
- Rybelsus is administered orally once daily, while liraglutide is administered via subcutaneous injections once daily
- The FDA has approved both drugs for the treatment of diabetes, however, only Liraglutide is FDA-approved for the treatment of obesity.
Rybelsus in a dose of 14 mg was compared with Liraglutide in a dose of 1.8 mg.
Significant weight loss was observed after 26 weeks in the Liraglutide and Rybelsus groups compared to the placebo.
| Baseline Weight | Weight after 26 weeks | Mean weight loss | |
| Placebo | 93.2 | 92.7 | -0.5 kgs |
| Liraglutide 1.8 mg | 95.5 | 92.4 | -3.1 kgs |
| Rybelsus 14 mg | 92.9 | 88.5 | -4.4 kgs |
The mean weight loss was significant in the liraglutide and Rybelsus groups compared with the placebo medicine.
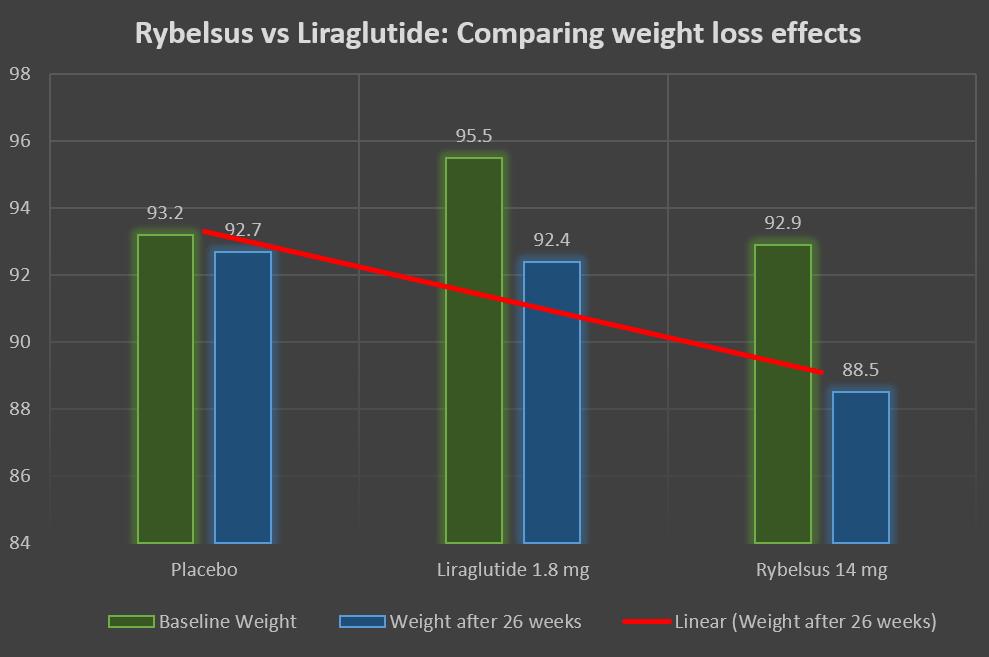
It can be observed here, that Rybelsus, although not FDA-approved for weight loss in non-diabetic patients, it seems superior to Liraglutide (an FDA-approved weight-loss drug).
Furthermore, Rybelsus is available as an oral pill. Thus, it may be preferred to Liraglutide which is an injectable medicine.
| You may also like to read: |
Rybelsus Vs Orlistat (Xenical):
Orlistat (Xenical) is another FDA-approved weight loss drug. It has been estimated in clinical trials to be associated with significant weight loss compared to a placebo.
Orlistat inhibits dietary fats. With orlistat, more calories in the form of dietary fats are lost from the gut.
It has been associated with weight loss ranging from 5 to 10%. In absolute terms, it may result in a weight loss of about 3 kg (6.6 lbs).
On the other hand, Rybelsus, although not approved as a weight loss drug, it is associated with greater weight loss compared to orlistat (Xenical).
Rybelsus is associated with a weight loss of about 3 to 4.5 kgs. This is slightly better than Orlistat as Orlistat only causes a weight loss of about 3 kg.
In summary:
Rybelsus has not been approved by the FDA for weight loss in non-diabetic patients, but it is a potential drug for obese individuals as an off-label medicine.
It seems superior to one of the FDA-approved weight-loss drugs, Liraglutide.
| You may also like to read: |