Saxenda or Victoza are two brand names of Liraglutide. Liraglutide is a GLP-1 analog that acts like endogenous incretins, stimulating insulin release and suppressing glucagon in a glucose-dependent mechanism.
Here we discuss the common and uncommon side effects of Saxenda and withdrawal symptoms (if any) that might occur once the drug is discontinued.
Can abrupt Saxenda discontinuation result in Withdrawal Symptoms?
Withdrawal symptoms are abnormal psychological or physical symptoms that may develop when a drug is abruptly discontinued.
Withdrawal symptoms are more common with medications that may cause physical or psychological dependence.
Dependence has not been reported with the use of Saxenda. However, patients may experience some of the following symptoms on abrupt discontinuation of Saxenda.
High blood glucose (Impaired glycemic control):
Since Saxenda is an antidiabetic medicine, the abrupt withdrawal will result in uncontrolled blood glucose. Patients may report blurring of vision, polyuria, nocturia, and dry mouth.
Binge eating and weight gain:
Since Saxenda suppresses the appetite, patients may notice an improvement in the gastrointestinal side effects associated with Liraglutide (Saxenda).
Thus, patients are likely to start eating more. This could result in weight gain.
It is important to note these Saxenda Withdrawal Symptoms are not actually withdrawal symptoms. For some patients, these symptoms could be a relief especially those with persistent GI side effects.
Others might develop weight gain and hyperglycemia and develop anxiety and palpitations which could be falsely labeled as withdrawal symptoms.
| You may also like to read: |
Saxenda Side effects and Contraindications:
Saxenda is contraindicated in patients with a personal or a family history of medullary thyroid carcinoma and MEN II syndrome.
It should also be avoided in pregnant females and individuals who are allergic to the drug.
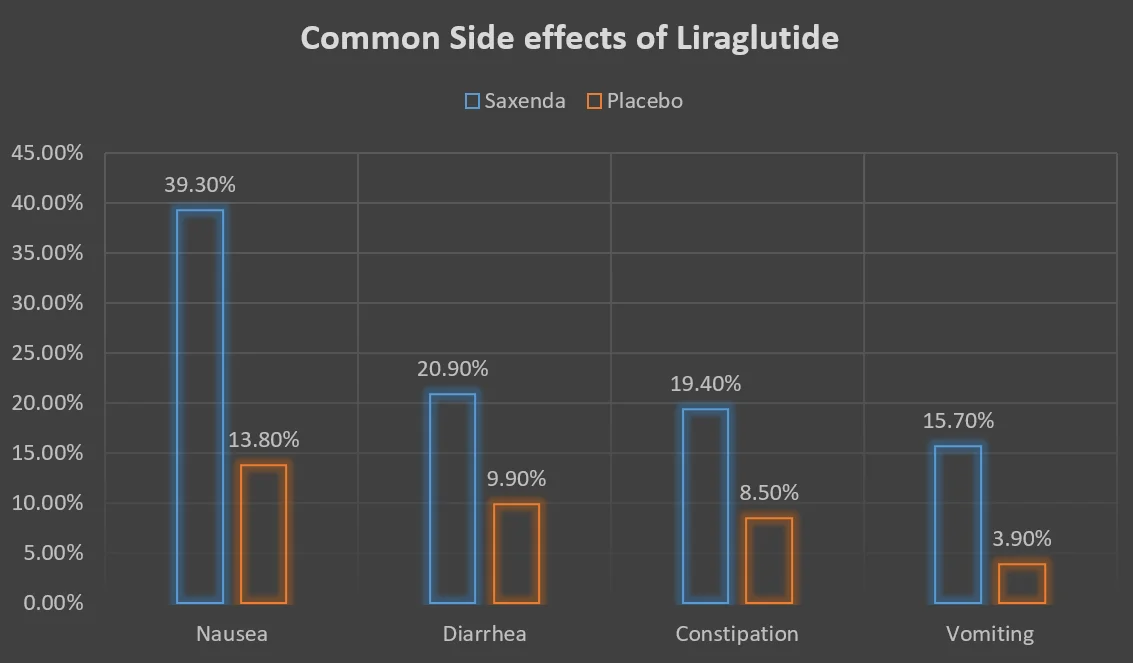
Common Side effects of Saxenda (Liraglutide) [Ref]:
Like all GLP-1 analogs, the most common side effects of Saxenda (Liraglutide) are related to the gastrointestinal system.
The most common side effects were nausea, diarrhea, constipation, and vomiting.
Common Side effects | Saxenda | Placebo |
| Nausea | 39.30% | 13.80% |
| Diarrhea | 20.90% | 9.90% |
| Constipation | 19.40% | 8.50% |
| Vomiting | 15.70% | 3.90% |
Other less common gastrointestinal side effects reported in the clinical trials were:
Other Gastrointestinal Side effects | Percentage reported |
| Dyspepsia | 9.6% |
| Abdominal Pain | 5.4% |
| Upper Abdominal Pain | 5.1% |
| Gastroesophageal Reflux Disease | 4.7% |
| Abdominal Distension | 4.5% |
| Eructation | 4.5% |
| Flatulence | 4.0% |
| Dry Mouth | 2.3% |
| You may also like to read: |
Hypoglycemia and reduced appetite:
Since Saxenda (Liraglutide) enhances the release of insulin and suppresses glucagon, it is used primarily to lower blood glucose.
In clinical trials, the incidence of hypoglycemia was greater in patients who were treated with Saxenda (liraglutide) compared to those treated with a placebo.
Among those patients who developed hypoglycemia, 3 patients developed severe hypoglycemia (and required the assistance of another person during the episode).
It was observed that all the patients who developed severe hypoglycemia were also receiving sulfonylureas, though, the dose of sulfonylurea was reduced by 50% at the beginning of the trial.
| Hypoglycemia in the active treatment group | Hypoglycemia in the Placebo group | |
| Saxenda + Sulfonylurea | 43.60% | 27.30% |
| Saxenda Monotherapy | 15.70% | 7.60% |
| You may also like to read: |
Acute Pancreatitis with Saxenda (Liraglutide):
Acute pancreatitis is the inflammation of the pancreas. It may manifest as upper abdominal pain, vomiting, and anorexia.
The pain is usually moderate to severe in intensity and is not relieved with OTC PPIs and analgesic medications.
In clinical trials, acute life-threatening hemorrhagic and non-hemorrhagic pancreatitis have been observed with Saxenda use.
The risk is three times greater in patients treated with Saxenda compared to placebo medicine.
Gallstone may develop in patients treated with Saxenda:
Saxenda inhibits the motility of the gall bladder resulting in stasis of the enzymes and pigments in the gall bladder. Prolong stasis may result in gallstones. Furthermore, rapid weight loss may also play a role in the development of gallstones.
The risk of gallstones and subsequent cholecystitis is three times greater in patients treated with Saxenda compare to Placebo.
Most patients who develop symptomatic gallstones and cholecystitis required surgical intervention in clinical trials.
Patients with a history of gallstones should be initiated on Saxenda treatment with extreme caution.
| You may also like to read: |
Allergic reactions and Injection site reactions:
Injection site reactions may occur with Saxend injection. These were more commonly reported in the Saxenda-treated group compared to placebo (13.9 % vs 10.5%) in clinical trials. Redness, itching, and rash were the most commonly reported symptoms.
Generalized allergic reactions are less common, however, because of their severity, they can not be ignored.
0.7% of patients in the Saxenda group developed hypersensitivity reaction vs 0.5% in the placebo.
These reactions included facial swelling, shortness of breath, wheezing, anaphylactic reactions, bronchial hyper-reactivity, asthmatic reactions, hypotension, and anaphylactic reactions.
Miscellaneous Side effects of Saxenda:
Other unrelated generalized side effects include fatigue, asthenia, loss of taste and smell, lethargy, weakness, and dizziness.
These symptoms are more common in patients who have concomitant nausea, vomiting, and other gastrointestinal side effects.
Patients may develop antibodies against the drug. In clinical trials, 2.8% of the patients were noted to develop antibodies against the drug.
Out of the 2.8%, 1.2% were observed to develop neutralizing antibodies. These patients are more likely to develop allergic reactions at the site of the injection and may develop hypoglycemia.
Hypotension and cardiac conduction problems have been rarely reported with Saxenda therapy.
Similarly, patients treated with Saxenda are more likely to develop depression, suicidal thoughts, and behavior. It may result in a worsening of pre-existing psychiatric symptoms.
| You may also like to read: |
Can patients treated with Saxenda develop cancers?
The three cancers that have been studied in patients treated with Saxenda are:
- Breast Cancer
- Papillary Thyroid Cancer
- Colorectal Cancer
Breast Cancer Risk with Saxenda (Liraglutide):
- In clinical trials, out of 2379 Saxenda-treated patients, 0.6% developed breast cancer compared to 0.2% in the 1300 placebo-treated patients.
- Invasive breast cancer was reported in 11 patients treated with Saxenda compared to 2 patients in the placebo group.
- Most of these cancers were estrogen and progesterone-receptor-positive.
Papillary Thyroid Cancer risk with Saxenda (Liraglutide):
- 7 (0.2%) of the Saxenda-treated patients develop papillary thyroid cancer compared with none in the placebo group.
- These cancers were usually small (less than one cm).
Colorectal Cancer risk with Saxenda (Liraglutide):
- 17 (0.5%) of 3291 Saxenda-treated patients were observed to develop colorectal cancer compared with 4 (0.2%) of 1843 placebo-treated patients.
- 2 patients (0.1%) of these developed invasive colorectal cancer compared to none in the placebo-treated group.
| You may also like to read: |
Laboratory abnormalities reported with Saxenda:
Commonly reported abnormal laboratory findings with the use of Saxenda include:
- An abnormal elevation of Liver Functions (Alanine aminotransferase or SGPT and Aspartate aminotransferase or SGOT) has been reported.
- Patients may develop hyperamylasemia (elevated amylase and lipase levels). The significance is not known but these patients may be at risk of developing pancreatitis.
- Serum Calcitonin levels were more likely to be elevated in patients who were treated with Saxenda compared to placebo in clinical trials.
- Patients who develop the clinical features of thyroid cancer such as neck swelling or lumps in the neck and those with significantly elevated calcitonin levels should be evaluated for medullary thyroid cancer.
Keywords used in this article:
- Saxenda withdrawal symptoms (Liraglutide withdrawal symptoms)
- Saxenda Side effects (Liraglutide side effects, Victoza Side effects)
- Saxenda and Cancer risk (Liraglutide cancer risk)
- Saxenda and medullary thyroid cancer
- Liraglutide and cancer risk
- Saxenda and pancreatitis (Liraglutide and Pancreatitis, Victoza and pancreatitis)
| You may also like to read: |




