Tirzepatide (Mounjaro), manufactured and marketed by Eli Lilly, has recently got approval from the FDA in May 2022 for the treatment of patients with Diabetes Mellitus Type 2.
Tirzepatide (Mounjaro) is the first in its class that has a dual incretin-mimetic effect. It is a dual GLP-1 (Glucagon-like peptide) and GIP (Glucose-dependent Insulinotropic peptide) agonist.
Previously, drugs that acted on the incretin pathway targeted GLP-1 receptors only. These drugs include Semaglutide (Ozempic), Dulaglutide (Trulicity), and Liraglutide (Victoza).
Update: Tirzepatide got FDA approval as a weight loss drug under the brand name, Zepbound
Tirzepatide (Mounjaro) Uses & Indications:
Tirzepatide is indicated for the treatment of adult patients with Diabetes Mellitus Type 2 in conjunction with a low-calorie diabetic diet and exercise.
Although it is superior to Semaglutide as a weight loss drug in clinical trials, it has not yet been approved for the treatment of obesity and weight loss.
In addition, like all GLP-1 mimetic drugs, the drug has not been approved for the treatment of children with diabetes (age younger than 18 years of age) and patients with Diabetes Mellitus Type 1.
Tirzepatide has also not been studied in patients with a history of pancreatitis.
Tirzepatide (Mounjaro) Dosage Forms [Ref]:
Tirzepatide is available as a single prefilled pen that contains 0.5 ml of clear to slightly yellow solution.
Every 0.5 ml of the prefilled pen comes in various strengths. The different dosage forms of Mounjaro are:
- 2.5 mg per 0.5 ml prefilled pen
- 5 mg per 0.5 ml prefilled pen
- 7.5 mg per 0.5 ml prefilled pen
- 10 mg per 0.5 ml prefilled pen
- 12.5 mg per 0.5 ml prefilled pen
- 15 mg per 0.5 ml prefilled pen
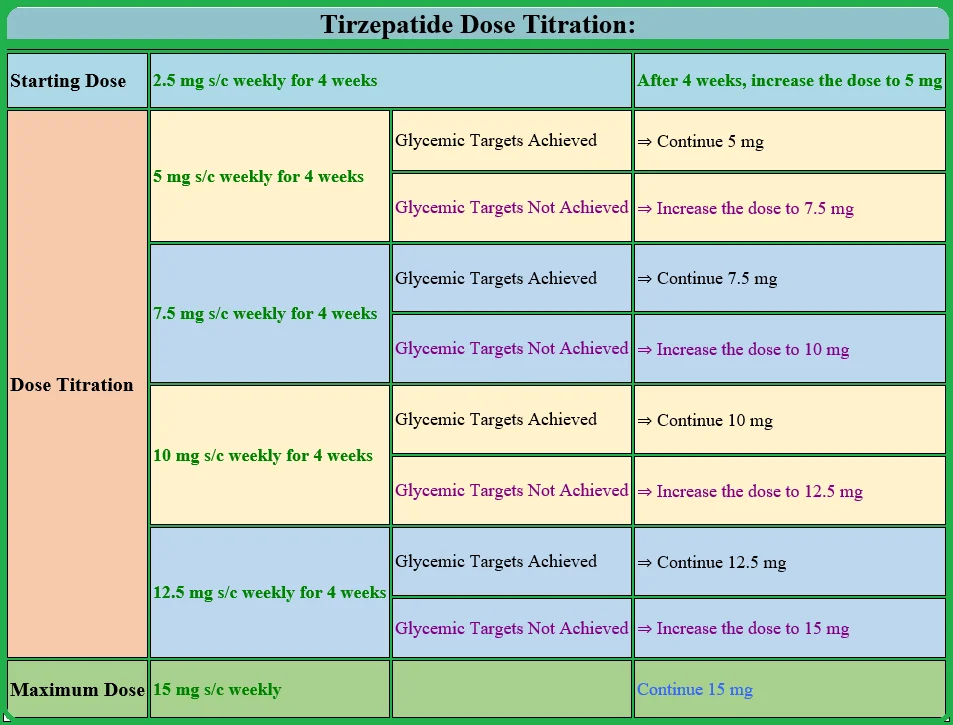
Tirzepatide (Mounjaro) Dose and Dosage Titration:
The starting dose of Mounjaro injection is 2.5 mg subcutaneously once a week for four weeks. It is important to note that the low dose (2.5 mg weekly) is not considered a diabetic dose.
This is the priming dose to see whether the patient can or will tolerate the dose or not. In addition, starting directly with a high dose may be associated with more gastrointestinal side effects.
After the patient tolerates the 2.5 mg weekly dose for four weeks, the dose is titrated upwards to 5 mg weekly.
Patients tolerating 5 mg well and achieving their glycemic targets may be continued with the 5 mg dose.
Those who need further blood-glucose-lowering effects may increase the dose by 2.5 mg weekly every four weeks to the maximum recommended weekly dose of 15 mg.
It is important not to exceed the maximum dose of 15 mg per week.
Tirzepatide Dose Titration:
| Starting Dose | 2.5 mg s/c weekly for 4 weeks | After 4 weeks, increase the dose to 5 mg | |
| Dose Titration | 5 mg s/c weekly for 4 weeks | Glycemic Targets Achieved | ⇒ Continue 5 mg |
| Glycemic Targets Not Achieved | ⇒ Increase the dose to 7.5 mg | ||
| 7.5 mg s/c weekly for 4 weeks | Glycemic Targets Achieved | ⇒ Continue 7.5 mg | |
| Glycemic Targets Not Achieved | ⇒ Increase the dose to 10 mg | ||
| 10 mg s/c weekly for 4 weeks | Glycemic Targets Achieved | ⇒ Continue 10 mg | |
| Glycemic Targets Not Achieved | ⇒ Increase the dose to 12.5 mg | ||
| 12.5 mg s/c weekly for 4 weeks | Glycemic Targets Achieved | ⇒ Continue 12.5 mg | |
| Glycemic Targets Not Achieved | ⇒ Increase the dose to 15 mg | ||
| Maximum Dose | 15 mg s/c weekly | Continue 15 mg | |
If a dose is missed, it can be administered as soon as possible and within four days of the scheduled time.
However, if more than four days have passed, one can either skip the dose and continue with the next dose at the scheduled time or administer the dose and continue with the new weekly schedule.
The day of the dosing schedule can be changed, but a gap of at least three days (72 hours) is recommended between two doses.
Also, if the dose is titrated up and the person develops severe side effects, the dose can be reduced to the last dose that was tolerated by the person.
For example, a person on 7.5 mg of Tirzepatide increases the dose to 10 mg. After switching to 10 mg, side effects start to develop (such as nausea, fullness, diarrhea/ constipation). The patient can switch back to the previous dose, i.e., 7.5 mg weekly.
The dose may be continued for a few months and may be given a try again after some months. However, if the side effects are very severe, the treatment may be discontinued.
Tirzepatide can be administered in elderly patients who are 75 years of age or more without altering the dose or frequency of the drug.
However, it has not been studied in children and should be avoided in diabetic patients who are younger than 18 years of age.
If overdosing occurs, supportive treatment in the form of hydration, antacids, and antiemetics may be advised. Patients should be monitored for at least one week since the half-life of the drug is 5 days.
How to Administer Tirzepatide (Mounjaro):
First of all, you should know that you can not use Tirzepatide (Mounjaro) if you are using any of the following diabetes medications:
- GLP-1 analogs such as:
- Ozempic (Semaglutide)
- Rybelsus (Semaglutide)
- Wegovy (Semaglutide)
- Trulicity (Dulaglutide)
- Victoza (Liraglutide)
- Saxenda (Liraglutide)
- Albiglutide
- Exenatide
- Or any other GLP-1 analog
- DPP-IV Inhibitors:
- Sitagliptin (Januvia)
- Vildagliptin (Galvus)
- Linagliptin (Trajenta)
- Saxagliptin (Onglyza), Or
- Any DPP-IV Inhibitor
Before injection Mounjaro, inspect the content of the injection. It should be very clear or slightly yellowish. Cloudy injection or one that contains any particulate matter must be discarded.
Mounjaro injection is available as a prefilled injection. It must be injected into the anterior aspect of the abdomen 3 to 5 cm away from the umbilicus.
Other skin areas where it can be injected include the skin of the thighs, the back of the upper arm, and the skin of the buttocks. However, it is best to administer it to the skin of the abdomen.
The injection technique is the same as an insulin injection. The needle is kept straight. A skin fold of the tummy is held between the thumb and the index finger of the left hand. The pen is held in the right hand with the thumb over the top.
After inserting the needle straight, with the thumb of your right hand, push the button and press it until all the contents of the pen are injected.
Wait for about 10 to 15 seconds and then remove the needle.
It is important not to mix Mounjaro with any other medicine or insulin. If the patient is on insulin and Mounjaro, their injection sites should be different.
You can inject insulin on the right side of the navel and Mounjaro on the left side of the navel.
Tirzepatide (Mounjaro) Use in Liver and Kidney Diseases:
Tirzepatide (Mounjaro) can be administered without dose adjustment in patients with liver or kidney disease.
It has been studied in patients with end-stage renal disease and patients with variable degrees of hepatic impairment. Liver and kidney dysfunction do not alter the pharmacokinetics of the drug.
However, it is important to watch for kidney function when escalating the dose and in patients who develop gastrointestinal side effects such as nausea, vomiting, and diarrhea.
Tirzepatide (Mounjaro) Use in Pregnancy and Breastfeeding:
Tirzepatide has not been studied in pregnant females. In animals, when administered during the period of organogenesis, it resulted in fetal growth retardation and fetal abnormalities.
Congenital abnormalities are common in newborns born to mothers with gestational diabetes (6 – 10%). The risk is much higher in mothers with glycated hemoglobin exceeding 7% (around 20 to 25%).
The primary goal should be focused on controlling diabetes. Preferred drugs like insulin and metformin should be used as the first line.
The manufacturer recommends using Tirzepatide only if the risks outweigh the benefits. However, this statement applies to any medicine that is not tested during pregnancy.
Since we have potent medications available for the treatment of gestational diabetes, we should avoid using Tirzepatide in pregnant females.
Similarly, Tirzepatide is not recommended to be used during lactation as data regarding its excretion into human breast milk is not sufficient to draw a conclusion.
Furthermore, Tirzepatide may impair gastric emptying. Delayed gastric emptying can alter the efficacy of oral contraceptive pills.
Patients must be advised to switch to other forms of contraception when using Mounjaro (Tirzepatide).
Tirzepatide (Mounjaro) Contraindications:
Tirzepatide is contraindicated in patients with:
- Serious allergic or hypersensitivity reactions to the drug
- Individuals with a personal or family history of MEN-2 syndrome or medullary thyroid carcinoma.
MEN-2 syndrome is further subdivided into two different types, MEN-2A and MEN-2 B.
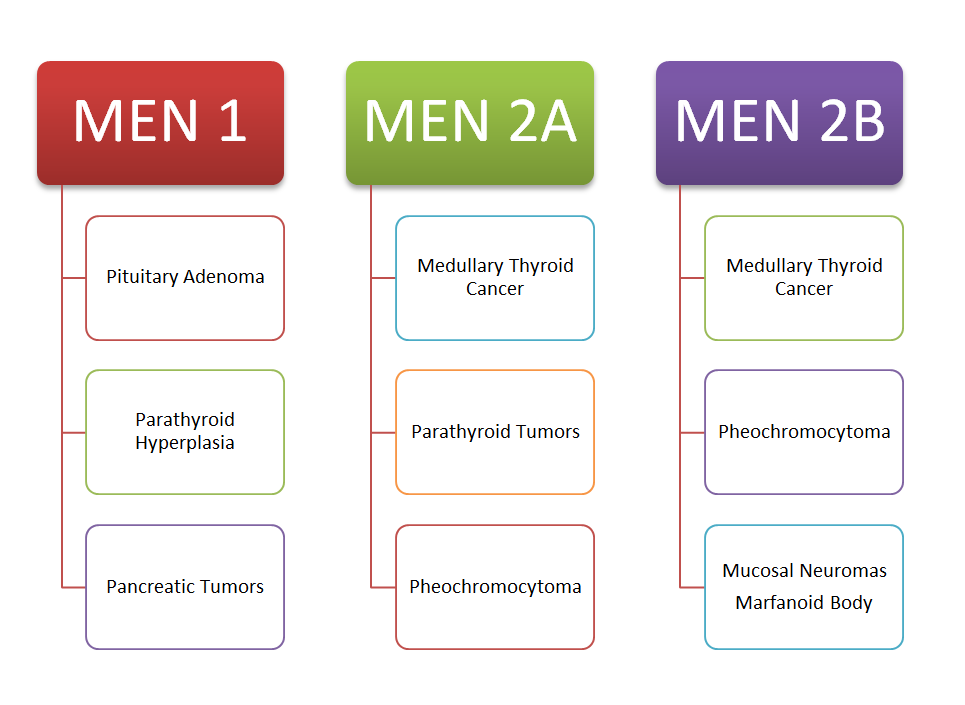
MEN-2A comprises Medullary thyroid carcinoma, parathyroid adenoma, and pheochromocytoma.
MEN-2B comprises Medullary thyroid carcinoma, pheochromocytoma, and mucosal neuromas.
Individuals with medullary thyroid cancer or MEN-2 syndrome should not use Tirzepatide or any GLP-1 analog.
Tirzepatide (Mounjaro) Side Effects and Warnings:
Medullary Thyroid Cancer:
Tirzepatide has been associated with medullary thyroid cancer in rats in a dose-dependent manner. The higher the dose, the greater the risk. In addition, the longer the duration of use, the greater the risk.
Hence, individuals who have a personal or family history of medullary thyroid cancer or MEN-2 syndrome should not use Tirzepatide.
All patients should be warned about the risk. Patients must be asked to report any swelling in the neck while on treatment.
Those who develop swelling, lymphadenopathy, hoarseness of voice, or difficulty in ingesting food should undergo an ultrasound of the neck and plasma calcitonin levels.
Levels exceeding 50 ng/L must be investigated for the occurrence of MTC. Routine monitoring of Calcitonin levels is not recommended because of the low incidence of the disease.
Pancreatitis:
Pancreatitis has been commonly associated with Tirzepatide compared to placebo or comparator diabetes drugs.
In clinical trials, out of 14 cases of pancreatitis, 13 cases occurred in patients who were using Tirzepatide versus one in the comparator group.
Pancreatitis, including fatal hemorrhagic pancreatitis, can occur. Patients must be warned to report any new onset of abdominal pain radiating to the back, nausea, and vomiting.
Patients who are suspected to have or those who have confirmed pancreatitis should stop the treatment.
In addition, it has not been studied in patients with a previous history of pancreatitis and hence it should be avoided in these patients.
Hypoglycemia:
Hypoglycemia is not a side effect of Mounjaro if it is given alone as monotherapy. However, when administered with insulin and especially with sulfonylureas, the risk is very high.
Patients who are initiated on Tirzepatide treatment must reduce their insulin and sulfonylurea doses to avoid developing low blood glucose.
Hypersensitivity reactions:
Hypersensitivity reactions can occur in patients on treatment. Mild allergic reactions may include localized or generalized urticaria or eczema.
However, severe hypersensitivity reactions have also been reported. These reactions may include angioedema, anaphylaxis, and shock.
Patients must be monitored for any kind of allergic reactions. Those with severe reactions should stop the treatment.
Treatment may be reinitiated in mild to moderate cases; however, in severe cases, treatment reinitiation may be too risky and should be avoided.
Acute kidney injury:
Acute kidney injury, new-onset or worsening of renal failure, has been reported in patients who are newly started on Tirzepatide treatment or those whose dose is escalated.
Renal failure has been commonly seen in patients with gastrointestinal symptoms. Nausea, vomiting, and diarrhea may result in volume depletion, resulting in kidney failure.
Patients who are initiated on treatment and those whose dose is escalated must be monitored for kidney injury.
Gastrointestinal side effects:
GI side effects, sometimes severe, are common in patients on Tirzepatide treatment. It is contraindicated in patients with gastroparesis and those who have severe gastrointestinal side effects.
Diabetic Retinopathy:
Like Ozempic and Insulin, rapid improvement in glycemic control has been associated with the worsening of diabetic retinopathy.
Mounjaro has not been studied in patients who have non-proliferative, proliferative diabetic retinopathy, or macular edema.
Patients started on treatment must be observed for worsening symptoms or new symptoms of diabetic retinopathy.
Gallbladder disease:
0.6% of the patients who received Tirzepatide treatment developed gallbladder disease compared to none in the placebo.
Acute gallbladder disease may occur in the form of a new onset of gallstones or cholecystitis that may require surgical intervention.
Other side effects (as reported in clinical trials):
Gastrointestinal side effects:
Gastrointestinal side effects were the most commonly reported side effects of Tirzepatide.
GI-related side effects occurred in 37.1%, 39.6%, and 43.6% of patients who were using Tirzepatide 5 mg, 10 mg, and 15 mg, respectively, compared with 20.4% in the placebo-treated group of patients [Ref].
The following gastrointestinal side effects were the most common:
Side effects | Mounjaro 5 mg(%) | Mounjaro 10 mg(%) | Mounjaro 15 mg(%) | Placebo(%) |
| Nausea | 12 | 15 | 18 | 4 |
| Diarrhea | 12 | 13 | 17 | 9 |
| Loss of appetite | 5 | 10 | 11 | 1 |
| Vomiting | 5 | 5 | 9 | 2 |
| Constipation | 6 | 6 | 7 | 1 |
| Dyspepsia | 8 | 8 | 5 | 3 |
| Abdominal pain | 6 | 5 | 5 | 4 |
The above adverse reactions occurred in at least 5% of the Mounjaro-treated patients.
Other commonly reported GI side effects that occurred in less than 5% of the treated patients include the following:
Side effects | Mounjaro 5 mg(%) | Mounjaro 10 mg(%) | Mounjaro 15 mg(%) | Placebo(%) |
| Eructation | 3 | 2.5 | 3.3 | 0.4 |
| Flatulence | 1.3 | 2.5 | 2.9 | 0 |
| GERD | 1.7 | 2.5 | 17 | 0.4 |
| Abdominal distension | 0.4 | 2.9 | 0.8 | 0.4 |
More patients in the treatment group opted out of the trial because of gastrointestinal side effects.
Compared to placebo (0.4%), 3% of patients in the Mounjaro 5 mg group, 5.4% in the 10 mg group, and 6.6% in the 15 mg group opted out of the trial because of GI-related side effects.
Hypoglycemia:
Hypoglycemia was not reported in the treatment group when the drug was given as monotherapy.
However, when added to basal insulin with or without metformin, 2 cases of severe hypoglycemia in the Mounjaro 10 mg and 1 case of severe hypoglycemia in the Mounjaro 15 mg were reported.
Severe hypoglycemia was more frequently reported when Mounjaro was co-administered with sulfonylurea.
Increase in the heart rate:
An average of 2 to 4 beats increase in the heart rate was noticed in the treatment group compared to 1 beat in the placebo-treated patients.
In Japanese patients, the increase in heart rate was more common.
Injection site reaction:
Injection-site reactions occurred commonly in patients treated with Mounjaro compared to placebo (3.2% vs 0.4%).
Injection-site reactions may include erythema, redness, pain, induration, and swelling at the injection site.
Laboratory abnormalities:
Non-significant elevations in serum amylase and lipase levels have been reported with its use. In asymptomatic patients, the clinical relevance of these abnormal laboratory tests has not been clearly defined.
However, patients must be monitored for pancreatitis.
Tirzepatide (Mounjaro) Monitoring Parameters:
Specific monitoring parameters have not been clearly defined. However, all patients must have blood glucose monitored to see their response to treatment.
Glycated Hemoglobin should be monitored every six months in patients who achieve their glycemic targets and three-monthly in patients with uncontrolled diabetes.
Amylase, Lipase, and other pancreatic tests may be performed based on the patient’s symptoms and condition.
Weight should be monitored in all patients, especially obese diabetic patients. Those patients who have significant weight loss may need to reduce the dose of the drug or of the concomitant drug, especially insulin and sulfonylureas.
Tirzepatide (Mounjaro) Mechanism of Action (MOA):
Tirzepatide is a dual GLP-1 and GIP receptor activator. It mimics the endogenous GLP-1 and GIP and activates these receptors.
The activation of these incretin receptors results in glucose-dependent insulin production. Simultaneously, it also affects glucagon secretion.
The activation of GLP-1 receptors suppresses the release of Glucagon, while GIP receptors causes the release of Glucagon.
However, Glucagon release is inhibited when the blood glucose is high. Similarly, Glucagon suppression via GLP-1 receptors is also inhibited when the blood glucose is low. Overall, Tirzepatide suppresses glucagon levels in a glucose-dependent manner.
The overall effects on blood glucose are:
- It lowers fasting as well as post-prandial blood glucose
- It enhances the first and second phases of insulin
- It enhances insulin sensitivity
- It reduces the fasting and postprandial glucagon concentration (as much as 28%)
- Tirzepatide delays gastric emptying; however, this effect fades over time.
- It also slows the absorption of food and hence reduces post-meal glucose spikes
- It suppresses satiety and food intake
- It reduces body weight in obese individuals
Steady-state concentration:
- The steady-state concentration is achieved in about four weeks after administering it weekly for four weeks.
Absorption:
- It has a mean bioavailability of 80% when administered subcutaneously, regardless of whether it is administered into the skin of the abdomen, thighs, or upper arms.
- After administering subcutaneously, the maximum plasma concentration is achieved in about 8 to 72 hours.
Protein-binding:
- More than 99% of the drug is bound to albumin.
Elimination:
- It has a half-life of 5 days.
Metabolism:
Tirzepatide is metabolized by three processes:
- The peptide bond is metabolized by proteolytic cleavage
- The fatty acid part is metabolized by Beta Oxidation, and
- Amide hydrolysis
Excretion:
It is excreted in urine and feces. However, the intact molecule is not found in feces or urine.
Tirzepatide Brand Names and Cost:
Tirzepatide is available by the brand name of Mounjaro as a prefilled injection.
The retail price of the Mounjaro injection has been set at $12,666 per year ($263 per injection).
- 97% Pure Berberine Powder – High-purity, plant-derived extract with a rich yellow color. Carefully processed and lab-tes…
- Naturally Bitter Taste – Berberine has a strong, naturally bitter flavor. Best enjoyed when mixed with smoothies, tea, c…
- 100g in Resealable Foil Pouch – Packaged in a premium aluminum pouch to protect from moisture and light, keeping the pow…

- 5 Delicious Flavors: Freeze-Dried Mango, Freeze-Dried Blueberry, Freeze-Dried Orange, Freeze-Dried Dragon Fruit & Freeze…
- Pure and Natural Ingredients: Our fruit powders are made without synthetic pesticides, GMOs, or harmful chemicals. Each …
- Health Benefits: Our carefully selected fruits are packed with antioxidants, vitamins, fiber, and digestive enzymes to s…

- 🌿 4 Tangy Citrus Flavors in One Pack: Enjoy a delicious variety of Orange, Lime, Lemon, and Kiwi powder – 5 sachets of e…
- 💧Easy to Mix & Refreshing: Just add to water, smoothies, sparkling drinks, or tea for a vibrant citrus kick. Dissolves i…
- 🛡️Rich in Vitamin C & Antioxidants: Made from real fruit powders, this mix offers a natural source of vitamin C to suppo…

- VARIETY PACK – Includes 5 delicious organic berry powder flavors: Freeze-Dried Goji Berry, Freeze-Dried Strawberry, Free…
- PURE INGREDIENTS – Made with 100% natural, freeze-dried berries and absolutely no added sugar, artificial ingredients, o…
- CONVENIENT PACKAGING – Contains 20 pre-portioned 5g packets (100g total), eliminating the need for measuring and ensurin…









