In a recent Semaglutide Heart Failure clinical trial published in NEJM, Semaglutide in a dose of 2.4 mg weekly resulted in a significant improvement in symptoms, physical limitation, obesity, and exercise tolerance in obese patients with heart failure and a preserved ejection fraction [Ref].
The trial (STEP-HFpEF Trial) included more than 500 participants who were 18 years old of age or older and met the following criteria:
- Left ventricular ejection fraction ≥45%,
- BMI ≥30,
- NYHA functional class II-IV symptoms,
- KCCQ-CSS <90,
- 6-minute walk ≥100m, and
- One or more of the following:
- Elevated left ventricular filling pressures
- Elevated natriuretic peptide levels with echocardiographic abnormalities, or
- Hospitalization for heart failure in the past year plus either treatment with diuretics or echocardiographic abnormalities.
Participants were excluded from the study if they had:
- More than 5 kg weight change in the past 3 months,
- Had diabetes (T1DM, T2DM, or gestational diabetes)
- Had Myocardial infarction, stroke, unstable angina, TIA, or hospitalization because of heart failure within the past 30 days
- History of bariatric (cardiometabolic surgery) or planned during the trial period
- History of GLP-1 use within the past 3 months
- Contraindications to GLP-1 including:
- Pancreatitis within the past 6 months or chronic pancreatitis
- A personal or family history of medullary thyroid cancer or MEN-2 syndrome
- End-stage kidney disease
| Read: |
Baseline Characteristics of the Semaglutide Heart Failure Trial:
The trial was conducted in 13 countries (a total of 96 sites). These included Argentina, Australia, Canada, Czech Republic, Germany, Denmark, Hungary, Israel, Netherlands, Poland, Spain, the United Kingdom, and the United States
The baseline characteristics of the study participants are tabulated below:
Characteristic | Value |
| Gender | Women (56.1%) |
| Ethnicity | White (95.8%) |
| Age (median) | 69 years |
| Body Weight (median) | 105.1 kg |
| BMI (median) | 37.0 |
| BMI ≥ 35 | 349 (66.0%) |
| KCCQ-CSS (median) | 58.9 points |
| 6-minute Walk Distance (median) | 320.0 m |
| CRP Level (median) | 3.8 mg/L |
| Left Ventricular Ejection Fraction (median) | 57.0% |
| NT-proBNP Level (median) | 450.8 pg/mL |
| History of Atrial Fibrillation | 275 (52.0%) |
| Hospitalized for Heart Failure (last 12 mo.) | 81 (15.3%) |
| NYHA Class II | 66.2% |
| NYHA Class III or IV | 33.8% |
| Beta-blockers | Most participants |
| Diuretics | Most participants |
| Renin–Angiotensin System Blockers | Most participants |
| Mineralocorticoid Receptor Antagonists | 34.8% |
| SGLT2 Inhibitors | 3.6% |
| Read: |
The outcome of the Semaglutide Heart Failure Trial:
The two primary outcomes of the Semagltuide heart failure trial at week 52 were:
- a change in the KCCQ-CSS (The Kansas City Cardiomyopathy Questionnaire Clinical Summary Score), and
- a change in body weight
Key secondary end-points included a change in the 6-minute walk distance at week 52 and CRP levels.
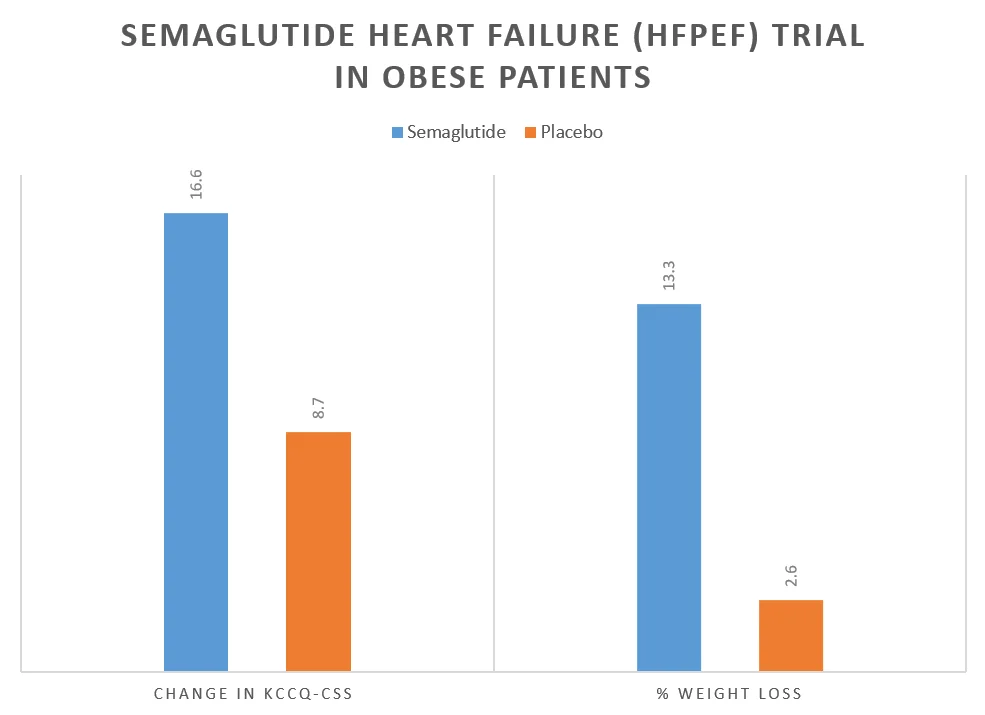
The results of the Semaglutide heart failure trial are tabulated below:
Change in End Points at 52 Weeks | Semaglutide | Placebo | Difference | P Value |
Dual Primary Endpoints | ||||
| Change in KCCQ-CSS score | 16.6 | 8.7 | 7.8 | <0.001 |
| % change in body weight | –13.3 | –2.6 | –10.7 | <0.001 |
Confirmatory Secondary Endpoints | ||||
| Improvement in 6-minute walk distance (m) | 21.5 | 1.2 | 20.3 | <0.001 |
| % Reduction in CRP level (%) | –43.5 | –7.3 | 0.61 | <0.001 |
The KCCQ-CSS (The Kansas City Cardiomyopathy Questionnaire Clinical Summary Score) is a 23-point score to assess the quality of life in patients with heart failure.
This score assesses the frequency, severity, and recent changes in heart failure-related symptoms, physical function, quality of life, and social function.
The score was transformed to 100 points ranging from 0 to 100 with higher scores indicating better quality of life compared to those with a low score.
Treatment Policy Estimand | KCCQ-CSS Mean Change | Body Weight % Change |
| Semaglutide Group | 16.6 points | -13.3% |
| Placebo Group | 8.7 points | -2.6% |
| Estimated Difference | 7.8 points | -10.7% |
| 95% Confidence Interval | (4.8 to 10.9) | (-11.9 to -9.4) |
| P-value | <0.001 | <0.001 |
The change in body weight ranged from 13.3% in the treatment policy estimand to 15.1% in the trial product estimand.
These two terms, treatment policy estimand, and trial product estimand are described as:
- Treatment policy estimand: The effect of the treatment on all participants regardless of the fact that the participants adhered to the treatment and were compliant with the treatment protocol or not.
- Trial product estimand: This estimates the effect of the drug in an ideal situation where the participants strictly adhered to the treatment protocol.
Trial Product Estimand | KCCQ-CSS Mean Change | Body Weight % Change |
| Semaglutide Group | 19.1 points | -15.1% |
| Placebo Group | 10.3 points | -2.4% |
| Estimated Difference | 8.8 points | -12.7% |
| 95% Confidence Interval | (5.9 to 11.7) | (-13.9 to -11.5) |
As can be seen from the tables above, Semaglutide resulted in a significant reduction in weight (13 – 15%) and improvement in heart failure-related symptoms as measured by the KCCQ-CSS score at week 52.
The authors of the study concluded that Semaglutide (Wegovy) in a dose of 2.4 mg once a week resulted in a greater improvement in the quality of life, exercise tolerance, and reduction in body weight in obese individuals with heart failure and a preserved ejection fraction (HFpEF).
| Read: |
What is Heart Failure with a preserved Ejection Fraction?
Heart failure with a preserved ejection fraction is a condition when the heart pumping ability is normal but because the heart muscles become stiff, it can not accommodate more blood.
This is also called diastolic heart failure. There is an increased filling pressure at the end of the systole.
One of the most common causes of “diastolic heart failure” or “heart failure with a preserved ejection fraction” is obesity.
Obesity is increasingly being recognized as one of the most important and reversible causes of HFpEF.
The proposed mechanisms of obesity-associated heart failure are:
- a decreased production of natriuretic peptides because the heart is unable to stretch adequately like normal people.
- Visceral adiposity can cause left ventricular hypertrophy and stiffness, insulin resistance, and arterial stiffness, in addition to its pro-inflammatory properties.
Other causes of heart failure with a preserved ejection fraction include:
- Hypertension
- Diabetes
- Aging
- Coronary artery disease and valvular heart disease
- Atrial fibrillation
- Chronic Inflammation, kidney disease, and chronic lung conditions
- Genetic Factors
- Anemia, and
- Sedentary lifestyle
The two most recently approved drugs for the management of diastolic heart failure or HFpEF are SGLT2 inhibitors and Sacubitril.
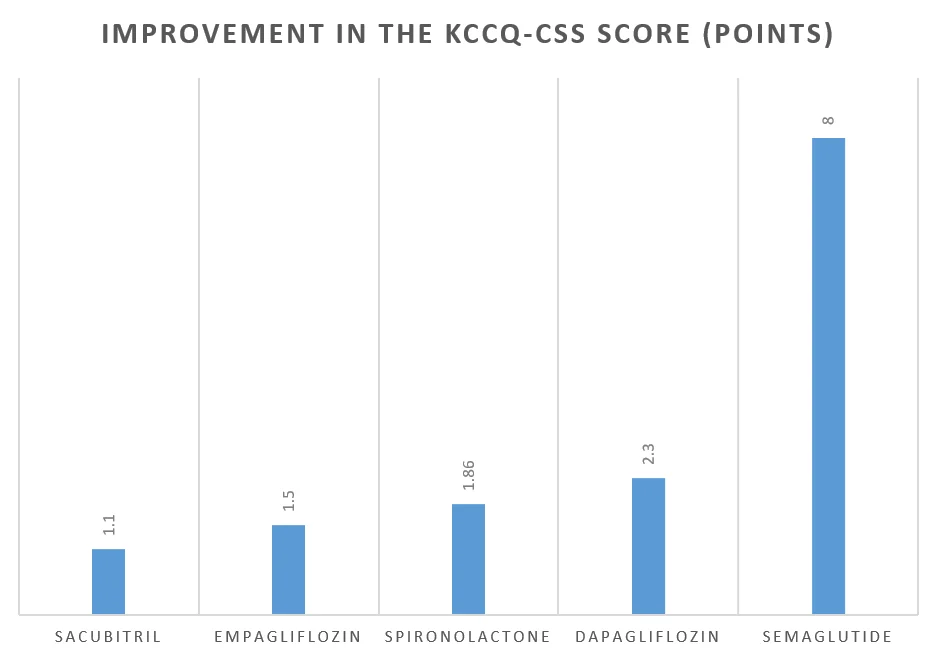
Compared to Semaglutide, these drugs had a modest increase in the KCCQ-CSS scores.
The table below shows the improvement in the quality of life as measured by the KCCQ-CSS score with these medicines:
Drugs approved for HFpEF | Increase in the KCCQ-CSS score (points) |
| Empagliflozin | +1.50 at 52 weeks [Ref] |
| Dapagliflozin | +2.3 at 34 weeks [Ref] |
| Spironolactone | +1.86 at 36 months [Ref] |
| Sacubitril | +1.1 at 34 weeks [Ref] |
| Semaglutide | +8 at 52 weeks [Ref] |
| Read: |
In Summary:
Semaglutide is a GLP-1 analog that has tremendous effects on the health of obese patients with heart failure. It lowers body weight and dramatically improves the quality of life in obese patients with heart failure.
The improvement in the quality of life is compared here with different drugs that have already been approved for the treatment of heart failure with a preserved ejection fraction.
Other formulations of Semaglutide (Ozempic and Rybelsus) have not been investigated in patients with heart failure and a preserved ejection fraction.
However, as per the ADA recommendations, they are considered the first-line in diabetics with ASCVD (atherosclerotic cardiovascular disease).
Lastly, Tirzepatide (Mounjaro) is considered one of the strong competitors of Semaglutide and has also not been studied in HFpEF.



