Tirzepatide Vs Semaglutide” compares the efficacy and safety of Tirzepatide and Semaglutide in individuals with type 2 diabetes and obesity.
Tirzepatide is a dual GLP-1 and GIP analog, also called Twincretin, while Semaglutide is a GLP-1 analog.
Tirzepatide is a newly approved drug for the treatment of type 2 diabetes and obesity.
For Diabetes, it is marketed by Eli Lilly as Mounjaro. For weight loss, it is marketed as Zepbound.
In clinical trials, it has been associated with significant weight loss. However, its approval as an obesity drug is still pending.
Mechanism of Action of Incretins: GLP-1 and GIP:
The two most common incretins that affect glucose and lipid metabolism, and regulate gut motility and appetite are GLP-1 (Glucagon-like peptide 1) and GIP (Glucose-dependent insulinotropic polypeptide).
GLP-1 analogs include:
- Exenatide (Byetta),
- Liraglutide (Victoza and Saxenda),
- Dulaglutide (Trulicity), and
- Semaglutide (Rybelsus, Ozempic, and Wegovy).
These drugs cause the release of insulin in a glucose-dependent mechanism and simultaneously suppress glucagon levels.
Unlike GLP-1 analogs, GIP is glucagonotropic in states where the blood glucose is low or normal. It increases the release of glucagon in states of hypoglycemia or normal blood glucose levels.
GIP (Glucose-dependent insulinotropic polypeptide) is thought to potentiate the central appetite suppressant effect of GLP-1 analogs. Furthermore, the receptors for GIP are present in abundance in the adipose tissues.
Thus, GIP activation improves post-prandial hyperglycemia by increasing the sensitivity of the adipose tissues to insulin.
It also enhances the lipid-buffering capacity of white adipose tissue, resulting in the prevention of ectopic fat deposition.
What is Tirzepatide (Mounjaro)?
Tirzepatide is a newly approved drug for the treatment of type 2 diabetes and weight loss. It has a dual mechanism of action.
It acts as an incretin-mimetic. However, unlike most of the currently available drugs that primarily target GLP-1, Tirzepatide targets both GLP-1 and GIP.
Tirzepatide is structurally similar to the endogenous Glucose-dependent insulinotropic polypeptide and includes a C20 fatty acid moiety.
Like the latest GLP-1 analogs, it has a long half-life of about five days. It is administered subcutaneously once a week.
Tirzepatide Vs Semaglutide:
Tirzepatide is available by the brand names of Mounjaro and Zepbound, while Semaglutide is available as Rybelsus, Ozempic, and Wegovy.
There are subtle differences in their onset and duration of action. These similarities and differences are highlighted in the table below:
Tirzepatide | Semaglutide | |
| Brand Names | Mounjaro |
|
| Class | Dual GLP-1 and GIP analog | GLP-1 analog |
| Formulation | Injection |
|
| Dose | 2.5 mg, 5 mg, 10 mg, and 15 mg |
|
| Administration | Subcutanoues |
|
| Maximum Plasma concentration | 8 – 72 hours |
|
| Duration of action | 28 days | 28 days |
| Half-life | 5 days | 5 days |
The SURPASS Trial (A Study of Tirzepatide [LY3298176] versus Semaglutide Once Weekly as Add-on Therapy to Metformin in Participants with Type 2 Diabetes) evaluated the safety and efficacy of the two drugs in a head-to-head trial [Ref].
The study was conducted on patients with type 2 diabetes who had inadequate control on metformin monotherapy.
Patients received either Semaglutide 1 mg weekly subcutaneously or Tirzepatide in a dose of 5 mg, 10 mg, or 15 mg subcutaneously once a week.
Efficacy of Tirzepatide Vs Semaglutide:
Tirzepatide was compared in the SURPASS Trial in a head-to-head comparison with Semaglutide.
It was observed that the glycated hemoglobin was significantly reduced in patients who were treated with Tirzepatide compared to Semaglutide in a dose-dependent manner.
The HbA1C reduced at week 40 from the baseline by 2.01%, 2.24%, and 2.30% with Tirzepatide 5 mg, 10 mg, and 15 mg compared to Semaglutide 1 mg weekly (1.86%)

Drug | A1C reduction | Efficacy Vs 1 mg Semaglutide |
| Semaglutide 1 mg | 1.86% | – |
| Tirzepatide 5 mg | 2.01% | 1.08 times more effective |
| Tirzepatide 10 mg | 2.24% | 1.2 times more effective |
| Tirzepatide 15 mg | 2.30% | 1.24 times more effective |
A greater percentage of the patients in the Tirzepatide group achieved the target HbA1C at week 40 compared to Semaglutide. This is demonstrated here: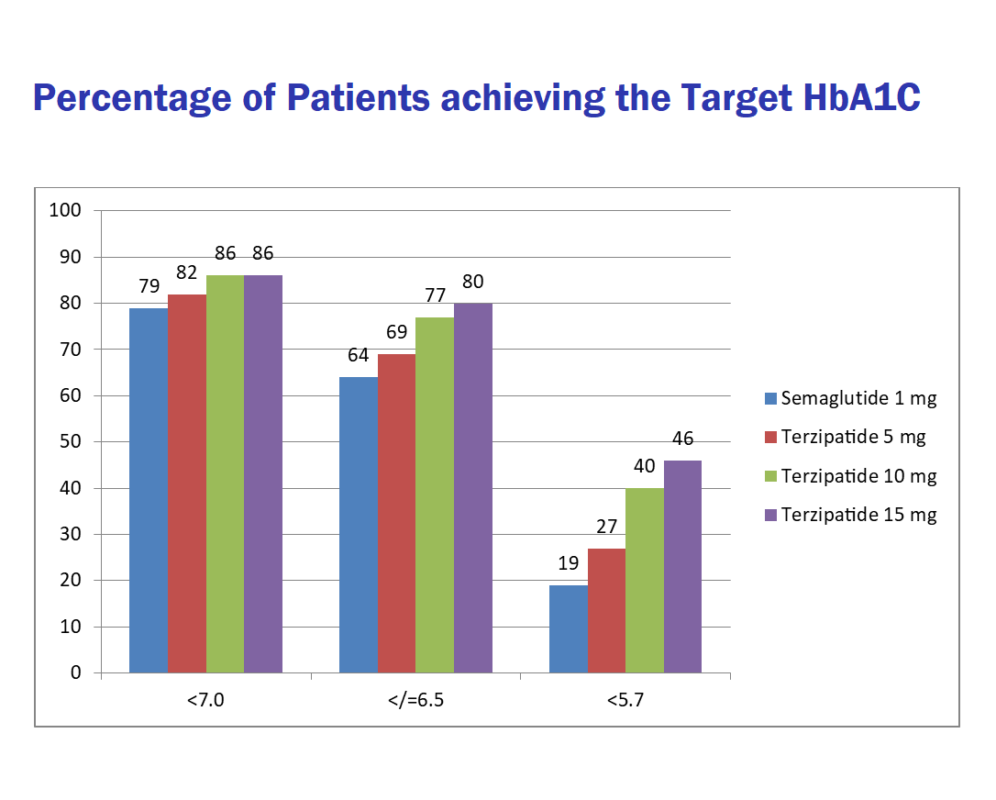
Percentage of patients in each group achieving the target glycated hemoglobin:
Drugs | A1C < 7% | A1C < 6.5% | A1C < 5.7% |
| Semaglutide 1 mg | 79% | 64% | 19% |
| Tirzepatide 5 mg | 82% | 69% | 27% |
| Tirzepatide 10 mg | 86% | 77% | 40% |
| Tirzepatide 15 mg | 86% | 80% | 46% |
Comparing Weight Loss Effects of Tirzepatide Vs Semaglutide:
All GLP-1 analogs have been associated with weight loss. The recent STEPS trials demonstrated marked weight loss with a high dose of Semaglutide 2.4 mg administered once weekly.
Based on the data from the STEPs Trials, it got FDA Approval. The maximum weight loss that occurred in the Wegovy (high-dose Semaglutide 2.4 mg once a week) group was 16%.
The SURPASS Trial compared the 1 mg Semaglutide (Ozempic) administered once a week compared to Tirzepatide at different doses.
It was observed that more weight loss occurred in the Tirzepatide group compared to patients in the 1 mg Semaglutide group.
A weight loss of 5.7 kgs occurred in the Semaglutide group compared to 7.6 kgs, 9.3 kgs, and 11.2 kgs in the Tirzepatide 5 mg, 10 mg, and 15 mg respectively.

Drugs | Weight Loss | Times Tirzepatide is More Effective |
| Semaglutide 1 mg | 5.7 kgs | – |
| Tirzepatide 5 mg | 7.6 kgs | 1.3 |
| Tirzepatide 10 mg | 9.3 kgs | 1.63 |
| Tirzepatide 15 mg | 11.2 kgs | 1.96 |
A greater percentage of patients achieved the target weight loss of 5 kgs, 10 kgs, and 15 kgs in the Tirzepatide group compared to patients in the Semaglutide group.
This is demonstrated here:

Drugs | >5% weight loss | >10% weight loss | >15% weight loss |
| Semaglutide 1 mg | 54% | 24% | 8% |
| Tirzepatide 5 mg | 65% | 34% | 15% |
| Tirzepatide 10 mg | 76% | 47% | 24% |
| Tirzepatide 15 mg | 80% | 57% | 36% |
Other effects of Tirzepatide Vs Ozempic:
Apart from the greater reduction in the HbA1C and significant weight loss of Tirzepatide compared to Semaglutide, it was observed that patients in the Tirzepatide group had a greater improvement in their lipid profiles compared to those treated with Semaglutide.

Adverse drug reactions were slightly higher in the Tirzepatide group and were dose-dependent compared to Semaglutide.
Most patients had adverse events related to GI symptoms. These included nausea, vomiting, diarrhea, dyspepsia, constipation, flatulence, reduced appetite, and abdominal pain.
The most common adverse event was nausea and was reported in 17.4%, 19.2%, and 22.1% in the Tirzepatide 5 mg, 10 mg, and 15 mg respectively, compared to 17.9% in the Semaglutide group.
In Conclusion:
The Novel Drug, Tirzepatide, is a promising drug for the treatment of Diabetes and obesity. It is the first drug that targets both GLP-1 and GIP, resulting in greater glycemic control and appetite suppression.
Other drugs which are in clinical trials are:
- 97% Pure Berberine Powder – High-purity, plant-derived extract with a rich yellow color. Carefully processed and lab-tes…
- Naturally Bitter Taste – Berberine has a strong, naturally bitter flavor. Best enjoyed when mixed with smoothies, tea, c…
- 100g in Resealable Foil Pouch – Packaged in a premium aluminum pouch to protect from moisture and light, keeping the pow…

- 5 Delicious Flavors: Freeze-Dried Mango, Freeze-Dried Blueberry, Freeze-Dried Orange, Freeze-Dried Dragon Fruit & Freeze…
- Pure and Natural Ingredients: Our fruit powders are made without synthetic pesticides, GMOs, or harmful chemicals. Each …
- Health Benefits: Our carefully selected fruits are packed with antioxidants, vitamins, fiber, and digestive enzymes to s…

- 🌿 4 Tangy Citrus Flavors in One Pack: Enjoy a delicious variety of Orange, Lime, Lemon, and Kiwi powder – 5 sachets of e…
- 💧Easy to Mix & Refreshing: Just add to water, smoothies, sparkling drinks, or tea for a vibrant citrus kick. Dissolves i…
- 🛡️Rich in Vitamin C & Antioxidants: Made from real fruit powders, this mix offers a natural source of vitamin C to suppo…

- VARIETY PACK – Includes 5 delicious organic berry powder flavors: Freeze-Dried Goji Berry, Freeze-Dried Strawberry, Free…
- PURE INGREDIENTS – Made with 100% natural, freeze-dried berries and absolutely no added sugar, artificial ingredients, o…
- CONVENIENT PACKAGING – Contains 20 pre-portioned 5g packets (100g total), eliminating the need for measuring and ensurin…








