Baxdrostat is a new medicine that is being investigated for the treatment of resistant hypertension. The results of the phase 2 clinical trial have been published in NEJM.
Treatment-resistant hypertension is defined as inadequate blood pressure control despite three antihypertensives of different classes that also include one diuretic drug.
A mineralocorticoid receptor inhibitor, such as Spironolactone is the usual fourth drug that is added to the treatment regimen. However, despite the addition of Spironolactone, half of the patients still have inadequate control (>130/80).
In addition, spironolactone is associated with gynecomastia and hyperkalemia, which may limit its use in men and patients with renal impairment at risk of hyperkalemia.
What is Baxdrostat?
Baxdrostat inhibits the enzyme aldosterone synthase, which is responsible for converting angiotensin II to Aldosterone.
It does not bind to the Mineralocorticoid receptor; rather, it inhibits the formation of aldosterone. Baxdrostat has 100 times more affinity to the enzyme aldosterone synthase vs the enzyme required for cortisol synthesis.
The diagram here shows the enzyme responsible for the conversion of angiotensinogen to angiotensin I, angiotensin II, and Aldosterone.
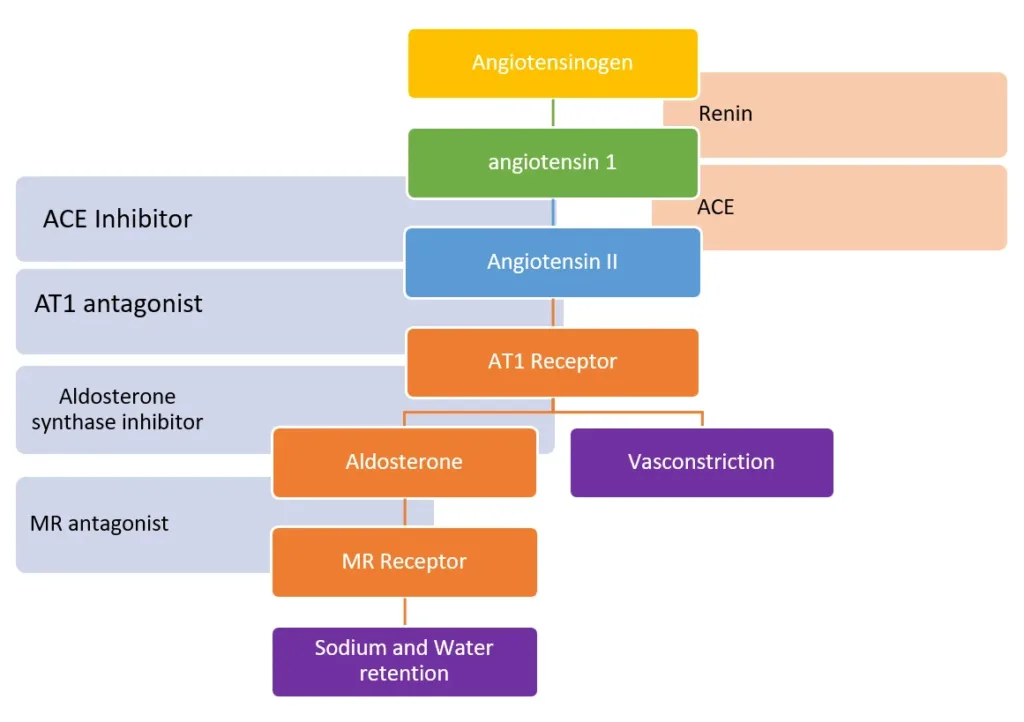
Various drugs have been approved for the treatment of hypertension. The most important of these are drugs that target the RAAS (Renin-angiotensin-aldosterone-system) pathway.
The most commonly used inhibitors of the RAAS pathway that are currently being used are:
- ACE-Inhibitor
- ARBs (Angiotensin receptor blockers)
- Renin-inhibitors
- Mineralocorticoid receptor inhibitors
Aldosterone synthase inhibitors are a new class of drugs that are not yet approved but seem to be very effective for the treatment of resistant hypertension.
How effective is Baxdrostat for treatment-resistant hypertension?
A total of 274 patients with treatment-resistant hypertension were studied. They were divided into the following four groups [Ref].
Intervention | Number of participants |
| Placebo | 69 |
| Baxdrostat 0.5 mg | 69 |
| Baxdrostat 1 mg | 69 |
| Baxdrostat 2 mg | 67 |
Their baseline systolic blood pressures were as follows:
| Intervention group | Mean systolic blood pressure | Mean diastolic blood pressure |
| Placebo | 148.9 | 88.2 |
| Baxdrostat 0.5 mg | 147.6 | 87.6 |
| Baxdrostat 1 mg | 147.7 | 87.7 |
| Baxdrostat 2 mg | 147.3 | 88.2 |
Most patients were using a Calcium-channel blocker, a diuretic, a beta-blocker, an ACE-inhibitor, or an ARB.
There was a significant drop in blood pressure in patients who were on Baxdrostat treatment vs placebo. In addition, the drop in blood pressure was dose-dependent.
Here is a summary of the blood pressure drop after Baxdrostat treatment after 12 weeks:
| Intervention | Mean blood pressure (systolic/ diastolic) before treatment | Mean blood pressure (systolic/ diastolic) after treatment | Mean improvement in blood pressure (systolic/ diastolic) with treatment |
| Placebo | 148.9/88.2 | 139.5/79 | -9.4/9.2 |
| Baxdrostat 0.5 mg | 147.6/87.6 | 135.5/79 | -12.1/8.6 |
| Baxdrostat 1 mg | 147.7/87.7 | 130.2/75.9 | -17.5/11.8 |
| Baxdrostat 2 mg | 147.3/88.2 | 127/73.9 | -20.3/14.3 |
It can be observed that the target blood pressure of 130/80 or less was observed in patients who received higher doses of Baxdrostat.
There were a few side effects noted in the treatment group. These included:
- Urinary tract infection
- Hyperkalemia
- Headache, and
- Fatigue
Most side effects were very mild, and some were unrelated to the drug. The most serious side effect was urosepsis.
In addition, three important side effects noted were:
- One case of hypotension
- Three cases of hyponatremia, and
- Six cases of Hyperkalemia
No cases of adrenal insufficiency were observed.
Plasma aldosterone levels dropped in a dose-dependent manner. Similarly, a compensatory increase in renin levels was also noted.
In conclusion:
Baxdrostat is a selective aldosterone synthase inhibitor. It inhibits the production of aldosterone, which is the key hormone in the RAAS pathway.
It was found in this phase 2 trial to be highly effective in lowering the blood pressure of patients with treatment-resistant hypertension who were already on three or more antihypertensive medications.
The drug was well tolerated; however, urinary tract infection, fatigue, headache, hyperkalemia, and some other common side effects were observed in the trial.
- 97% Pure Berberine Powder – High-purity, plant-derived extract with a rich yellow color. Carefully processed and lab-tes…
- Naturally Bitter Taste – Berberine has a strong, naturally bitter flavor. Best enjoyed when mixed with smoothies, tea, c…
- 100g in Resealable Foil Pouch – Packaged in a premium aluminum pouch to protect from moisture and light, keeping the pow…

- 5 Delicious Flavors: Freeze-Dried Mango, Freeze-Dried Blueberry, Freeze-Dried Orange, Freeze-Dried Dragon Fruit & Freeze…
- Pure and Natural Ingredients: Our fruit powders are made without synthetic pesticides, GMOs, or harmful chemicals. Each …
- Health Benefits: Our carefully selected fruits are packed with antioxidants, vitamins, fiber, and digestive enzymes to s…

- 🌿 4 Tangy Citrus Flavors in One Pack: Enjoy a delicious variety of Orange, Lime, Lemon, and Kiwi powder – 5 sachets of e…
- 💧Easy to Mix & Refreshing: Just add to water, smoothies, sparkling drinks, or tea for a vibrant citrus kick. Dissolves i…
- 🛡️Rich in Vitamin C & Antioxidants: Made from real fruit powders, this mix offers a natural source of vitamin C to suppo…

- VARIETY PACK – Includes 5 delicious organic berry powder flavors: Freeze-Dried Goji Berry, Freeze-Dried Strawberry, Free…
- PURE INGREDIENTS – Made with 100% natural, freeze-dried berries and absolutely no added sugar, artificial ingredients, o…
- CONVENIENT PACKAGING – Contains 20 pre-portioned 5g packets (100g total), eliminating the need for measuring and ensurin…









