Aimovig Vs Ajovy is a comparison between the two novel migraine medicines, Erenumab vs Fremanezumab.
There are no studies available to directly compare the efficacies and safety of the two drugs. Both the drugs belong to the same class of medicines and are very effective in the preventive treatment of migraine headaches.
Similarities and Differences between Aimovig and Ajovy:
Aimovig is the brand name of Erenumab while Ajovy is the brand name of Fremanezumab. As the names of these two drugs suggest, both these drugs are human monoclonal antibodies.
These antibodies target primarily affect the CGRPR and are used to prevent the recurrence and severity of migraine headaches.
Both Aimovig and Ajovy are available in the form of prefilled injections that can be administered monthly (or three-monthly in the case of Ajovy) subcutaneously.
These injection formulations can result in injection-site reactions and hence one of the common side effects of these two drugs is local pain, redness, swelling, and induration at the site of the injections.
The following table summarizes the differences between the Aimovig and Ajovy:
Similarities | Aimovig Vs Ajovy |
| What are they? | Both are human monoclonal antibodies |
| MOA | Both Aimovig and Ajovy target the CGRPR. |
| Uses | Both Aimovig and Ajovy are used in the preventive treatment of migraine headaches. |
| Form | Both Aimovig and Ajovy are available as single prefilled injections or autoinjectors. |
| Efficacy | Both Aimovig and Ajovy have been proven in clinical trials to reduce the number of migraine headache days. |
| Administration | Both the drugs are administered as a subcutaneous injection. |
| Storage | Both the injections should be stored in a refrigerator. It can be left for about 7 days without a refrigerator. |
| Common Side effects | Both Ajovy and Aimovig are associated with injection site reactions. |
| Patients assistance programs | Both the manufacturers provide Copay assistance plans by which the prices can be lowered.An online discount card is available for both Aimovig and Ajovy |
Aimovig Vs Ajovy: Key differences between the two:
Minute differences exist between the two CGRPR inhibitors.
Brand and Generic Names:
Aimovig is the brand name of Erenumab while Ajovy is the brand name of Fremanezumab.
Manufacturer:
Amgen pharmaceuticals are the primary manufacturers and marketers of Aimovig injections while Teva pharmaceutical is marketing Ajovy injections.
Dosing Frequency:
Both Ajovy and Aimovig injections can be administered monthly, however, Ajovy can also be administered every three months, depending on the dose of the drug. 225 mg is administered monthly while 675 mg is administered three monthly.
The usual recommended dose for Aimovig is 70 mg monthly. However, some patients may require higher doses of 140 mg monthly.
Peak plasma concentration and Half-life elimination:
It takes 5 – 7 days for Ajovy to reach maximum plasma concentration after subcutaneous administration while 6 days for Aiomvig. The half-life elimination of Ajovy is 31 days while that of Aimovig is 28 days.
Safety and Side effect profile:
In terms of safety, both drugs are quite safe with minimal systemic side effects. Local injection site reactions can occur with both drugs. However, systemic side effects such as constipation, muscle spasms, and pain have only been observed with Aimovig.
Cost Comparison:
The cost of both drugs is almost the same with minimal difference. Ajovy is 9 USD costlier than Aimovig.
These differences are summarized in the table below:
Aimovig | Ajovy | |
| Generic | Erenumab | Fremanezumab |
| Manufacturer | Amgen | Teva |
| Dosing | Aimovig is administered in a dose of 70 mg monthly injection into the skin. The dose can be increased in some patients to 140 mg per month. | Ajovy can be either administered monthly or three-monthly.The monthly dose is 225 mg subcutaneously while the three-monthly dose is 675 mg subcutaneously |
| Side effects | Although both the drugs are associated with injection site reactions, Aimovig also causes constipation | Constipation has not been reported with Ajovy |
| Latex allergy | Aimovig injection pen is made of natural rubber latex. Individuals allergic to latex can not use Aimovig. | In contrast to Aimovig, Ajovy is not made of a Latex device and hence can be safely used by people who are allergic to Latex. |
| Price | One prefilled injection of 70 or 140 mg/ml is USD 630 | One prefilled injection of 225 mg/1.5 ml costs USD 639 |
Efficacy of Aimovig Vs Ajovy: Which one is better?
Both Ajovy and Aimovig have been shown to be superior to placebo in reducing the number of migraine episodes and migraine days per month.
Efficacy of Ajovy in Clinical Trials [Ref]:
Ajovy (Fremanezumab) was studied in two groups of patients:
- Those with Episodic Migraine, and
- Those with Chronic Migraine
Episodic migraine headache was defined as patients with migraine headaches of fewer than 15 days per month.
Chronic migraine headache was defined as those with 15 or more days of migraine headaches.
Ajovy in Episodic Migraine Headache:
Ajovy was studied in patients with episodic migraine. Patients without cardiovascular diseases, thrombotic diseases, or cerebrovascular diseases were divided into three different groups:
- Placebo
- Ajovy 225 mg per month
- Ajovy 675 mg three-monthly
The primary end-point was a reduction in the number of migraine days after three months. The secondary end-point was a 50% reduction in the number of migraine days after three months.
The results of the study are tabulated here:
Efficacy Endpoints | Placebo | Ajovy 225 mg monthly | Ajovy 675 mg three-monthly |
Primary end-point (Reduction in monthly migraine days) | |||
| Baseline Migraine Days | 9.1 | 8.9 | 9.2 |
| Change from Baseline | -2.2 | -3.7 | -3.4 |
| Difference from Placebo | – | -1.5 | -1.2 |
| Significance (P-value) | – | Yes (<0.001) | Yes (<0.001) |
Secondary endpoint (Greater than 50% reduction in the number of migraine days) | |||
| % Responders | 27.9% | 47.7% | 44.4% |
As seen in the table above, more patients in the Ajovy group had a reduction in the number of migraine days. Almost half of the patients had a 50% reduction in their number of migraine days when on Ajovy compared to placebo.
Ajovy in Chronic Migraine Headache:
In the treatment of chronic migraine, defined as the number of migraine days equal to or greater than 15 days per month, Ajovy resulted in significant improvement.
Patients without cardiovascular, thrombotic, or cerebrovascular diseases were enrolled in the study and followed after three months.
The end-points of the study were similar to the first study:
- The primary end-point was a reduction in the number of migraine days (of at least moderate severity) per month.
- The secondary end-point was a 50% reduction in the number of migraine days per month (of at least moderate severity.
The results of the study are summarized in the table below:
Efficacy Endpoints | Placebo | Ajovy 225 mg monthly | Ajovy 675 mg three-monthly |
Primary endPoint (Reduction in monthly migraine days) | |||
| Baseline Migraine Days | 20.3 | 20.3 | 20.4 |
| Change from Baseline | -2.5 | -4.6 | -4.3 |
| Difference from Placebo | -2.1 | -1.8 | |
| Significance (P-value) | <0.001 | <0.001 | |
Secondary endpoint (Greater than 50% reduction in the number of migraine days) | |||
| % Responders | 18.1% | 40.8% | 37.6% |
A significant reduction in the number of migraine days and the percentage of patients with a 50% reduction in the number of migraine days was observed in the Ajovy group compared to Placebo medicine.
The Efficacy of Aimovig in the preventive treatment of Migraine:
Aimovig (Erenumab) was studied in patients with episodic and chronic migraine. The definitions of episodic and chronic migraine were essentially similar to those in the Ajovy clinical trials:
Episodic migraine was defined as fewer than 15 migraine days per month (Migraine headache days of 4 to 14 days per month).
Chronic migraine headache was defined as greater than 15 migraine days per month.
A total of three studies evaluated the efficacy of Aimovig in episodic and chronic migraine headaches.
The efficacy of Aimovig in Episodic Migraine:
Two studies were done to evaluate the efficacy of Aimovig in episodic migraine. One study was of three months duration, while the other one was of six months duration.
In both studies, Aimovig resulted in significant clinical improvement and a reduction in the number of migraine headache days compared to placebo.
Study 1: A multicenter randomized study:
The first study was a 6-month follow-up study that evaluated the efficacy of 70 mg and 140 mg of Aimovig in patients with episodic migraine headaches.
All patients with cerebrovascular diseases, cardiovascular diseases, and thrombotic disorders were excluded from the study.
The results of the study are tabulated below:
Efficacy Endpoints | Placebo | Aimovig 70 mg once a month | Aimovig 140 mg once a month |
The primary endpoint (Reduction in monthly migraine days) | |||
| Change from Baseline | -1.8 | -3.2 | -3.7 |
| Difference from Placebo | -1.4 | -1.9 | |
| Significance (P-value) | <0.001 | <0.001 | |
Secondary endpoint (Greater than 50% reduction in the number of migraine days) | |||
| % Responders | 26.6% | 43.3% | 50% |
Aimovig resulted in a significant reduction in the number of migraine days compared to placebo. In addition, more patients in the Aimovig group observed a 50% reduction in their number of migraine days.
Study 2: A three-month randomized study:
Study 2 evaluated the efficacy of 70 mg of Aimovig in the treatment of patients with episodic migraine. Patients were followed after three months.
The results of the study are tabulated below:
Efficacy Endpoints | Placebo | Aimovig 70 mg once a month |
The primary endpoint (Reduction in monthly migraine days) | ||
| Change from Baseline | -1.8 | -2.9 |
| Difference from Placebo | -1.0 | |
| Significance (P-value) | <0.001 | |
Secondary endpoint (Greater than 50% reduction in the number of migraine days) | ||
| % Responders | 29.5% | 39.7% |
As noted, after three months, Aimovig resulted in a clinically significant reduction in the number of migraine days and alleviating symptoms.
Aimovig efficacy in the treatment of chronic migraine headache:
The efficacy of Aimovig was demonstrated in a three-month clinical trial in patients with chronic migraine. Chronic migraine was defined as the number of migraine headache days that are equal to or exceed 15 days per month.
The results of the study are tabulated below:
Efficacy Endpoints | Placebo | Aimovig 70 mg once a month | Aimovig 140 mg once a month |
The primary endpoint (Reduction in monthly migraine days) | |||
| Change from Baseline | -4.2 | -6.6 | -6.6 |
| Difference from Placebo | -2.5 | -2.5 | |
| Significance (P-value) | <0.001 | <0.001 | |
Secondary endpoint (Greater than 50% reduction in the number of migraine days) | |||
| % Responders | 23.5% | 39.9% | 41.2% |
Aimovig resulted in a clinically significant reduction in the number of migraine headache days. More patients in the Aimovig group had a 50% reduction in their number of migraine days compared to placebo.
It was also noted that patients in the Aimovig group used fewer acute migraine treatment medicines compared to those in the placebo group.
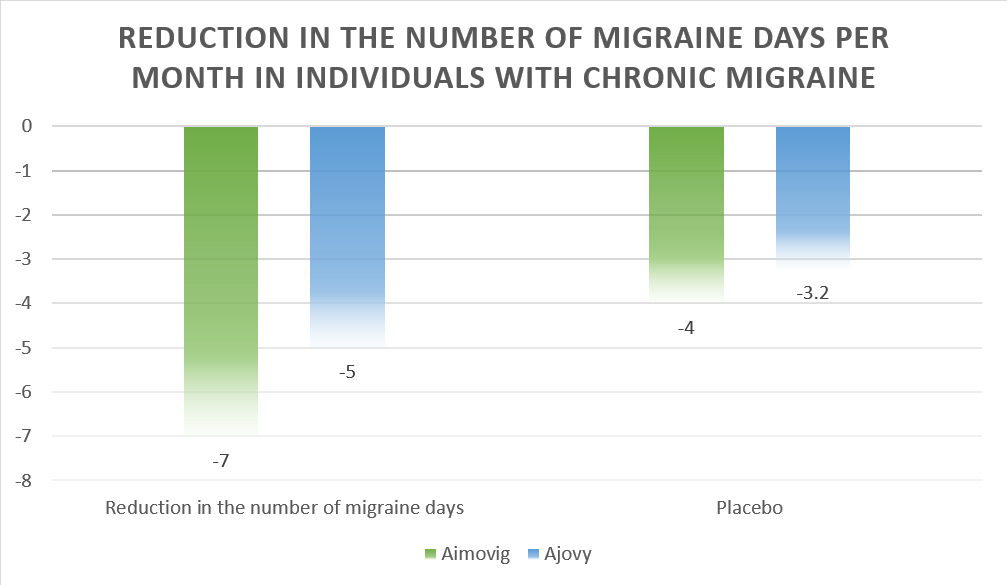
The efficacy of Aimovig vs Ajovy in Chronic Migraine:
As mentioned in the above clinical trials, both Ajovy and Aimovig resulted in clinically significant improvement in the symptoms of migraine headaches. This is depicted by the reduction in the number of migraine days per month.
Although the results of the study can not be compared directly, a summary of the efficacies of the drugs is presented below:
Aimovig | Ajovy | |
| Reduction in the number of chronic migraine days | -7 | -5 |
| Placebo | -4 | -3.2 |
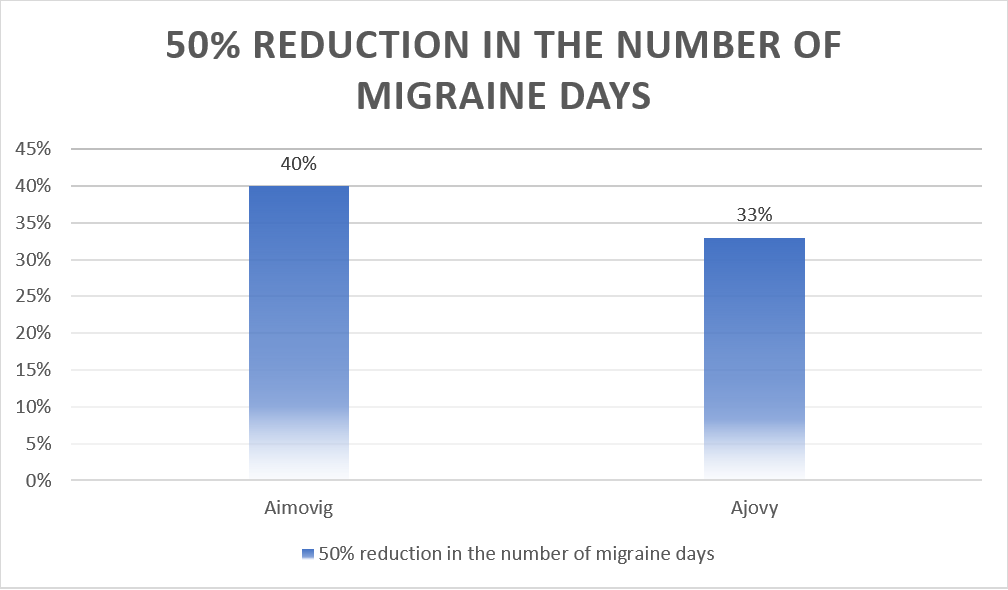
Similarly, patients in both the Aimovig and Ajovy groups had a 50% reduction in the number of migraine days compared to placebo.
| Drug | 50% reduction in the number of migraine days |
| Aimovig | 40% |
| Ajovy | 33% |
In conclusion:
Both Ajovy and Aimovig can be used in the preventive treatment of migraine headaches. Both drugs are quite safe and effective in reducing the frequency and severity of migraine headaches.
No head-to-head clinical trials have been conducted to establish the superiority of one drug over another.
- 97% Pure Berberine Powder – High-purity, plant-derived extract with a rich yellow color. Carefully processed and lab-tes…
- Naturally Bitter Taste – Berberine has a strong, naturally bitter flavor. Best enjoyed when mixed with smoothies, tea, c…
- 100g in Resealable Foil Pouch – Packaged in a premium aluminum pouch to protect from moisture and light, keeping the pow…

- 5 Delicious Flavors: Freeze-Dried Mango, Freeze-Dried Blueberry, Freeze-Dried Orange, Freeze-Dried Dragon Fruit & Freeze…
- Pure and Natural Ingredients: Our fruit powders are made without synthetic pesticides, GMOs, or harmful chemicals. Each …
- Health Benefits: Our carefully selected fruits are packed with antioxidants, vitamins, fiber, and digestive enzymes to s…

- 🌿 4 Tangy Citrus Flavors in One Pack: Enjoy a delicious variety of Orange, Lime, Lemon, and Kiwi powder – 5 sachets of e…
- 💧Easy to Mix & Refreshing: Just add to water, smoothies, sparkling drinks, or tea for a vibrant citrus kick. Dissolves i…
- 🛡️Rich in Vitamin C & Antioxidants: Made from real fruit powders, this mix offers a natural source of vitamin C to suppo…

- VARIETY PACK – Includes 5 delicious organic berry powder flavors: Freeze-Dried Goji Berry, Freeze-Dried Strawberry, Free…
- PURE INGREDIENTS – Made with 100% natural, freeze-dried berries and absolutely no added sugar, artificial ingredients, o…
- CONVENIENT PACKAGING – Contains 20 pre-portioned 5g packets (100g total), eliminating the need for measuring and ensurin…









