Retatrutide is a triple G agonist (GGG triagonist). It enhances the actions of GLP-1, GIP, and GLucagon. Thus, it is more or less the same as Mounjaro but with the addition of its action directly on GLucagon.
Retatrutide is a more potent GIP analog (about 8.9 times the endogenous GIP) and is a less potent GLP-1 (0.4 times the endogenous GLP-1) and Glucagon analog (0.3 times the endogenous glucagon).
Glucagon activation is a paradox. We know it is used as an antidote for insulin. It is clinically utilized in managing severe hypoglycemia in hospital settings.
How can glucagon activation be used to treat diabetes?
Similar to the mechanism of action of Mounjaro, Retatrutuide may also have effects on blood glucose only in certain glucose ranges. For example, the insulinotropic effects are stopped when the blood glucose falls to less than 100 mg/dl. At this point, glucagon activation is the dominant action of these drugs.
Henceforth, while Retatrutide may lower blood glucose when the plasma levels are high, it may prevent hypoglycemia when the blood glucose is low.
In addition, glucagon activates lipid metabolism and boosts metabolism. Thus, its use is associated with significant weight loss.
Retatrutide Weight Loss Trial:
In a phase 2 Trial, Retatrutide was studied at different doses in obese individuals who had a BMI of 30 kg/m² or more or who had a BMI of 27 kg/m² or more plus at least one obesity-associated medical condition (such as hypertension, diabetes, dyslipidemia).
Weight was monitored at 24 and 48 weeks. The table below summarizes the weight changes at 24 weeks:
Retatrutide Group | Dose (mg) | % Weight Change at 24 Weeks |
| Placebo | – | -1.6% |
| Retatrutide | 1 | -7.2% |
| Retatrutide | 4 | -12.9% |
| Retatrutide | 8 | -17.3% |
| Retatrutide | 12 | -17.5% |
Retatrutide 8 and 12 mg administered once weekly resulted in significant weight loss of more than 17% of the baseline.
At 48 weeks, the weight loss was even more significant. Individuals who used Reatrutide 8 and 12 mg lost more than 20% of their baseline weight. Here is a table summarizing the weight loss effects of Retarutide at different doses.
Retatrutide Group | Dose (mg) | % Weight Change at 48 Weeks |
| Placebo | – | -2.1% |
| Retatrutide | 1 | -8.7% |
| Retatrutide | 4 | -17.1% |
| Retatrutide | 8 | -22.8% |
| Retatrutide | 12 | -24.2% |
Here is a graphic presentation of the percentage weight loss from baseline at different doses:
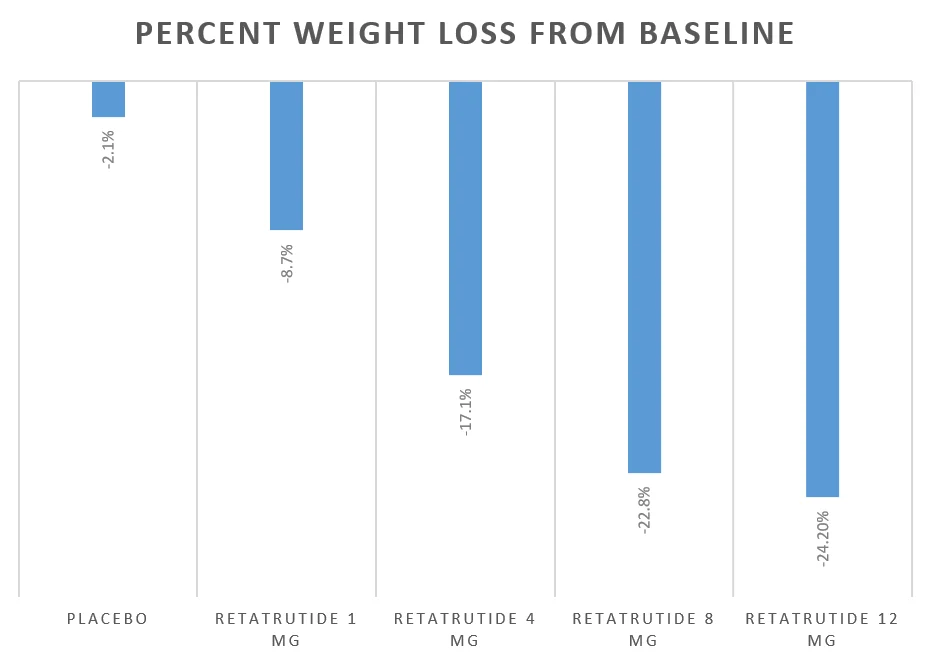
Retatrutide 1 mg and 4 mg resulted in modest weight loss, however, individuals who were on 8 and 12 mg lost 22.8% and 24.2% weight, respectively.
All the participants in the 8 and 12 mg dose groups lost 5% of their baseline body weight. In addition, more than 90% lost at least 10% of their baseline body weight.
The following table summarizes the results:
| Drug | Weight Loss ≥ 5% | Weight Loss ≥ 10% | Weight Loss ≥ 15% |
| Placebo | 27% | 9% | 2% |
| Retatrutide 4 mg | 92% | 75% | 60% |
| Retatrutide 8 mg | 100% | 91% | 75% |
| Retatrutide 12 mg | 100% | 93% | 83% |
Here is a graphic presentation of the above findings:

In addition to its weight loss effects, it was also observed that Retatrutide reverted prediabetes in 72% of the participants in the study after 48 weeks, vs 22% in the placebo group after 48 weeks.
30 to 41% of participants who were on more than one antihypertensive medication had to reduce the dose and discontinue one blood pressure-lowering medicine after 48 weeks [Ref].
Retatrutide Diabetes Trial:
Retatrutide was also investigated in a phase 2 Trial in the US in individuals with diabetes. The study included adult participants who had an A1C of 7 to 10.5% (53 – 91.3 mmol/l) and a BMI of 25 to 50 kg/m² were enrolled in the study.
Retatrutide showed significant improvements in A1C and body weight. Here is a table summarizing the A1C-lowering effects of Retatrutide.
| Treatment Group | Mean % Change in HbA1c at 24 Weeks | Mean Change in HbA1c (mmol/mol) |
| Placebo | -0.01% | -0.12 mmol/mol |
| Retatrutide 0.5 mg | -0.43% | -4.68 mmol/mol |
| Retatrutide 1.5 mg | -1.41% | -15.40 mmol/mol |
| Retatrutide 4 mg (escalation) | -1.39% | -15.24 mmol/mol |
| Retatrutide 4 mg | -1.30% | -14.20 mmol/mol |
| Retatrutide 8 (slow escalation) | -1.99% | -21.78 mmol/mol |
| Retatrutide 8 (fast escalation) | -1.88% | -20.52 mmol/mol |
| Retatrutide 12 mg escalation | -2.02% | -22.07 mmol/mol |
It is obvious from the table above that Retatrutide resulted in a dose-dependent, clinically significant reduction in the A1C after 36 weeks compared to placebo.
Similarly, the weight-lowering effects were also evaluated after 36 weeks. Up to 16% body weight loss occurred in the high-dose Retatrutide group vs only 3% in the placebo group.
Here is a table summarizing the weight loss effects of Retatrutide:
| Treatment Group | Bodyweight Change at 36 Weeks |
| Placebo | 3.00% |
| Retatrutide 0.5 mg | 3.19% |
| Retatrutide 1.5 mg | 2.02% |
| Retatrutide 4 (escalation) | 7.92% |
| Retatrutide 4 mg | 10.37% |
| Retatrutide 8 (slow escalation) | 16.81% |
| Retatrutide 8 (fast escalation) | 16.34% |
| Retatrutide 12 (escalation) | 16.94% |
In another trial, which was primarily intended to treat obesity, Retatrutide resulted in up to 24% weight loss vs placebo medicine.
To Conclude:
Retatrutide may be another blockbuster medicine for treating diabesity as it has shown superior results in obese and diabetic individuals vs placebo.
It has got a unique mechanism of action as it combines the agonistic effects of GLP-1 analogs (Ozempic, Trulicity, and Victoza), the agonistic effects of GIP and GLP-1 analog (Mounjaro), and the Glucagon-like effects.
Because it targets blood glucose and appetite at multiple levels, it is probably going to become one of the most potent diabetes and weight loss drugs.
- 97% Pure Berberine Powder – High-purity, plant-derived extract with a rich yellow color. Carefully processed and lab-tes…
- Naturally Bitter Taste – Berberine has a strong, naturally bitter flavor. Best enjoyed when mixed with smoothies, tea, c…
- 100g in Resealable Foil Pouch – Packaged in a premium aluminum pouch to protect from moisture and light, keeping the pow…

- 5 Delicious Flavors: Freeze-Dried Mango, Freeze-Dried Blueberry, Freeze-Dried Orange, Freeze-Dried Dragon Fruit & Freeze…
- Pure and Natural Ingredients: Our fruit powders are made without synthetic pesticides, GMOs, or harmful chemicals. Each …
- Health Benefits: Our carefully selected fruits are packed with antioxidants, vitamins, fiber, and digestive enzymes to s…

- 🌿 4 Tangy Citrus Flavors in One Pack: Enjoy a delicious variety of Orange, Lime, Lemon, and Kiwi powder – 5 sachets of e…
- 💧Easy to Mix & Refreshing: Just add to water, smoothies, sparkling drinks, or tea for a vibrant citrus kick. Dissolves i…
- 🛡️Rich in Vitamin C & Antioxidants: Made from real fruit powders, this mix offers a natural source of vitamin C to suppo…

- VARIETY PACK – Includes 5 delicious organic berry powder flavors: Freeze-Dried Goji Berry, Freeze-Dried Strawberry, Free…
- PURE INGREDIENTS – Made with 100% natural, freeze-dried berries and absolutely no added sugar, artificial ingredients, o…
- CONVENIENT PACKAGING – Contains 20 pre-portioned 5g packets (100g total), eliminating the need for measuring and ensurin…